Abstract
Urinary tract infections (UTIs) are primarily caused by uropathogenic Escherichia coli (UPEC), which frequently lead to recurrent infections. These bacteria utilize several strategies to establish infection in the host; in particular, virulence factors such as fimbriae and α-hemolysin facilitate persistent infection, evade host immune responses, and minimize antibiotic exposure. To date, antibiotics have been the primary treatment for UTIs. However, an increasing emphasis has been placed on the need for UTI vaccines, with mucosal vaccine products now available in several countries. Additionally, vaccines targeting intracellular UPEC, utilizing adjuvants, are currently under development. Understanding the pathogenic mechanisms of uropathogens has enabled the development of new treatment approaches, paving the way for next-generation preventive and therapeutic methods that could effectively manage recurrent UTIs in the future.
-
Keywords: Urinary tract infections, Bacterial infections, Persistent infection, Vaccines, Vaccine adjuvants
HIGHLIGHTS
Uropathogenic Escherichia coli (UPEC) uses fimbriae and α-hemolysin to establish infection, persist, evade immunity, and resist antibiotics. (2) Understanding pathogenic mechanisms has led to innovative urinary tract infection therapies. Noval vaccines target intracellular UPEC, using advanced adjuvants for better efficacy.
INTRODUCTION
Urinary tract infections (UTIs) are the second most common bacterial infection in humans, with a 2019 report indicating approximately 405 million cases and 267,000 deaths worldwide [
1]. In terms of gender, the incidence rate is reported to be about 35 times higher in women aged 16–35 compared to men [
2]. Around half of all women experience a UTI at least once in their life-time [
3], and in the United States, UTIs are associated with an estimated 2.9 million Emergency Department visits and 3.5 million outpatient consultations annually [
4,
5]. Despite treatment with antibiotics, UTIs are known to recur frequently, with many cases recurring within 6 months of treatment. Recurrent cystitis is diagnosed when it occurs at least twice within 6 months or at least 3 times within 1 year [
6–
8]. Additionally, up to 25% of patients presenting with UTI at clinics are estimated to have a history of prior UTI [
9]. Given that UTIs pose a significant threat to human health and life expectancy, thorough research into their pathogenesis is crucial for developing appropriate treatment strategies.
Various studies investigating the causes of recurrent cystitis have found that persistent pathogen infection is a major factor contributing to recurrent UTIs. Previous studies have reported that in 50% of patients with recurrent UTIs, the same bacterial strain responsible for the initial infection is involved in recurrence, and this bacterium can survive in the bladder for up to 3 years [
6,
8,
10]. Notably, studies using animal models have shown that the formation of intracellular bacterial communities (IBCs) is a form of persistent infection, and IBCs have also been observed in the tissues of patients with recurrent cystitis [
11,
12]. In addition to the virulence factors of pathogens, the failure of protective immune cells against infected host epithelial cells plays a key role in triggering recurrent cystitis [
13]. A deeper understanding of host-pathogen interactions will contribute to a more comprehensive understanding of UTIs and ultimately play an important role in preventing recurrent infections in patients. Particularly for patients experiencing recurrent cystitis, careful use of antibiotics is necessary, making the design of therapeutics based on pathogenicity crucial. Such approaches could help prevent the progression of UTIs to urosepsis and address the issue of antibiotic resistance, marking significant progress in treatment.
DEVELOPMENT OF PERSISTENT INFECTION BY UPEC
Uropathogenic
Escherichia coli (UPEC) is responsible for over 80% of UTIs in patients without underlying health conditions [
9]. In cases of complicated UTIs, this proportion decreases to 65%, yet UPEC remains the predominant pathogen. Other major bacteria known to cause UTIs include
Klebsiella pneumoniae,
Proteus mirabilis,
Staphylococcus species, group B
Streptococcus, and
Pseudomonas aeruginosa [
14]. In UPEC-induced UTIs, adhesive fimbriae serve as the most crucial virulence factor. Additionally, metal ion acquisition systems that allow UPEC to survive in nutrient-poor environments such as urine are also vital, and most UPEC strains contain the relevant genes for these systems [
15]. Among the various virulence factors secreted by UPEC, hemolysin plays an important role in establishing persistent infection [
16,
17]. Upon invasion of the human urinary tract, UPEC first interacts with the urothelial cells, subsequently encountering components of the innate immune response, including macrophages, neutrophils, and other immune cells that provide antimicrobial defense [
18]. There is a need for a detailed discussion on how UPEC overcomes this sequence of host defense mechanisms to establish persistent infections, ultimately leading to recurrent cystitis. By elucidating the pathogenicity of persistent infections, this section aims to explore vaccine design and utilization strategies to address and prevent these infections effectively.
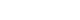
In most cases, UPEC approaches the urethra as fecal contaminants, eventually leading to bladder infection. During this process, UPEC must ascend the urethra to reach the bladder, although it is typically washed away by the periodic flow of urine. However, the type 1 fimbriae expressed on the surface of UPEC prevents this expulsion. Specifically, type 1 fimbriae bind strongly to the terminal mannosylated uroplakins, enabling UPEC not only to resist the initial clearance by urination but also to form colonies on the urothelial cells [
19]. Once adhered, UPEC proliferates rapidly in the bladder, and when its concentration in urine exceeds 1 × 10
5 CFU (colony forming unit)/mL, it is clinically diagnosed as a UTI [
20,
21].
Fimbriae are proteinaceous extracellular fibers located on the bacterial membrane, formed via the chaperone-usher pilus (CUP) system.
E. coli can encode up to 15 different types of CUP gene clusters, and the adhesive subunit at the tip of the fimbriae exhibits specificity for binding to extracellular surface molecules such as uroplakin on urothelial cells. The most extensively studied CUP fimbriae operon is fim, which encodes type 1 fimbriae. This fim operon is found in most UPEC isolates, and the adhesive subunit of type 1 fimbriae, FimH, binds to mannosylated glycoproteins, especially uroplakin, which are abundant on the bladder urothelial surface, allowing UPEC to establish itself in the bladder [
19]. The significantly lower bacterial loads observed in infected bladders with UPEC strains lacking FimH suggest that FimH is crucial for establishing infection [
22].
Among other CUP fimbriae, P fimbriae have been reported to directly contribute to kidney infections, suggesting that different types of fimbriae are associated with infections in specific organs [
23]. This is likely due to the enhanced ability of specific fimbriae to adhere to particular epithelial cell types expressed in each organ. Given the significant role of FimH in UTIs, several vaccines under development use FimH as an antigen.
After attaching to bladder epithelial cells (BECs) via FimH, a significant portion of UPEC remains on the cell surface to proliferate, while some invade the interior of the BECs. Unlike
Salmonella,
Yersinia, and
Shigella, which have a high capacity for invasion through the use of a type III secretion system, UPEC does not possess this system and therefore does not induce host cell plasma membrane ruffling or manipulate intracellular signaling through effector molecules. Instead, UPEC hijacks the recycling process of RAB27b
+ fusiform vesicles, which are necessary for BEC volume regulation, to gain entry into the host cells [
24,
25]. Once inside the BECs, UPEC can persist, reducing its exposure to antibiotics like ciprofloxacin, trimethoprim-sulfamethoxazole, and gentamicin, thereby facilitating recurrent infections [
26–
28].
Once internalized, UPEC can form and expand into IBCs within the host cytoplasm. In many cases, this process begins from a single bacterium that undergoes clonal expansion, resulting in bacterial clusters protruding into the bladder lumen, forming structures known as “pods” [
11]. UPEC within IBCs often undergoes morphological changes, typically adopting an elongated filamentous shape, which aids in escaping from the host cell and facilitates the infection of adjacent cells [
11,
29]. Notably, IBCs have been directly observed in desquamated epithelial cells from human bladder biopsy samples and urine [
12,
30]. The ability of intracellular UPEC to shield itself from antibiotics poses a significant challenge in eradicating persistent UPEC infections in patients with recurrent bladder infections. This makes it a critical issue in developing effective therapeutic strategies aimed at eliminating these persistent infections.
Studies on UTIs have revealed the existence of quiescent intracellular reservoirs (QIRs), which refer to clusters of UPEC encased in vesicles that persist within superficial epithelial cells for several weeks after epithelial exfoliation and regeneration have occurred. These QIRs remain dormant but can lead to recurrent infections if the epithelial cells become damaged [
26]. However, the exact conditions under which UPEC transitions from a QIR state to an IBC are still unclear.
Intriguingly, one study demonstrated that when urothelial cells infected with UPEC were treated with saponin, the total number of bacteria, both intracellular and extracellular, increased dramatically, and pod-like bacterial inclusions formed within the cells [
31]. This finding suggests that the transition from QIR to IBC may require environmental stimuli such as reactive oxygen species stress from neutrophils, the release of alarmins due to neighboring host cell death, abrupt changes in intracellular ion concentrations triggered by ion channel activation, or dysbiosis within the bladder’s microbial environment. These insights provide important clues into the mechanisms by which UPEC transitions from a dormant QIR state to an active IBC, further contributing to our understanding of recurrent UTIs and offering potential targets for therapeutic interventions.
Although genomic analyses of UPEC have shown that it is difficult to distinctly separate uropathogenic from non-uropathogenic strains [
32], the common toxins and their roles have been well studied. UPEC is known to secrete a variety of toxins during pathogenic infection [
33,
34], among which soluble toxins like α-hemolysin play a critical role. Several clinical studies have indicated that 40%–58% of UPEC isolates have the ability to secrete hemolysin, which is strongly correlated with UTI severity [
35–
39]. Interestingly, one clinical study reported that 37.6% of UPEC isolates from patients experiencing their first UTI expressed the
hlyA gene which encodes α-hemolysin, whereas this proportion increased to 48.2% in patients with recurrent UTIs [
40]. This suggests that α-hemolysin is closely associated with the severity of UTIs and may play a significant role in recurrent infections.
Alpha-hemolysin is secreted via the type I secretion system and has been shown to induce rapid cell death in urothelial cells during the early stages of infection [
34,
41–
43]. When UPEC secretes large amounts of hemolysin during the initial stages of infection, it forms channel-like structures on the cell surface, causing hemolysis and cytotoxicity, although this requires relatively high concentrations of hemolysin [
18,
44]. The plasma membrane appears to be a relatively favorable target for α-hemolysin compared to the vesicle membrane for several reasons. Firstly, the plasma membrane of host cells, composed of a lipid bilayer, maintains higher cholesterol levels and lower phosphatidylserine levels compared to vesicle membranes [
45]. Additionally, the plasma membrane is wider and flatter with lower curvature than vesicle membranes, making it more favorable for channel formation by hemolysin [
45]. Recent studies have reported that a receptor known as receptor A-disintegrin and metalloprotease 10 (ADAM10) may act as a scaffold for hemolysin channel formation, supporting the idea that external factors can influence this process [
46]. This suggests that hemolysin secreted by UPEC may form channels differently on the plasma membrane versus vesicle membranes.
Generally, α-hemolysin promotes damage to infected urothelial cells by inducing cytotoxicity, which leads to exfoliation of the bladder epithelium through caspase-1/ caspase-4-dependent pyroptosis [
34,
41–
43]. The role of α-hemolysin effectively explains the acute inflammation observed during the early stages of bacterial cystitis. Hemolysin expression and secretion are not random but are tightly regulated by environmental conditions. In fact, the CpxRA stress response system regulates the expression of hlyA. During the early inflammatory phase, hlyA expression is upregulated, while its expression is controlled as inflammation subsides, promoting bacterial persistence and recurrence of infection [
42]. This regulatory mechanism highlights how UPEC strategically uses α-hemolysin to initiate acute inflammation, while also ensuring its survival and the potential for recurrent infections through careful modulation of hemolysin expression.
The bladder, as an organ that stores urine, expands several times its size when filled compared to when it is empty. To accommodate this increase in volume, BECs require surface area expansion, which is facilitated by Rab27b
+ fusiform vesicles that fuse with the apical side of the bladder epithelium with the assistance of VAMP8/ Endobrevin [
25,
47]. When UPEC causes an infection, it hijacks this pathway to infiltrate the cells, remaining enclosed within Rab27b
+ vesicles after entering BECs. Although most of the UPEC is expelled from cells and cleared through urination due to the host's defense mechanisms, UPEC aided by hemolysin manages to escape from Rab27b
+ vesicles into the cytoplasm [
16]. Once in the cytoplasm, UPEC can establish a persistent infection. However, the host cell counters this by engaging the autophagy pathway, encapsulating UPEC in vesicles and ultimately attempting to inhibit bacterial proliferation and kill the pathogen through lysosomal vesicles. Despite this, many UPEC cells remain trapped within the LAMP1
+ lysosomal vesicles and fail to escape from the Rab27b
+ vesicles.
The persistence of UPEC within human BECs is closely associated with its ability to survive long-term inside LAMP1
+ lysosomal vesicles. Normally, lysosomal vesicles contain a variety of enzymes that require an acidic environment to be fully activated. However, it has been observed that vesicles in BECs infected by UPEC often maintain a neutral pH [
16]. This suggests that UPEC possesses the pathological capability to actively modulate the host cell environment, preventing the acidification of LAMP1
+ lysosomes [
16].
Phagosome acidification is facilitated by the V-ATPase proton pump, which resides on specific vesicle membranes. This pump is trafficked to the phagosome, where it fuses with the phagosome membrane and activates to acidify the compartment, creating optimal conditions for enzyme activity [
48,
49]. Typically, V-ATPase moves along microtubules to reach the necessary vesicles [
50–
52]. However, UPEC that escapes from RAB27b
+ vesicles into the cytoplasm secretes α-hemolysin, which disrupts microtubules [
16]. Infections with α-hemolysin-deficient UPEC mutants did not result in microtubule disruption when BECs were infected.
Further evidence of the importance of microtubule integrity came from experiments where colchicine, a microtubule-disrupting agent, was used to treat epithelial cells infected with these mutant strains. The treatment led to microtubule disassembly, significantly increasing the bacterial burden of intracellular UPEC [
16]. This indicates that vesicle trafficking, including the movement of V-ATPase, is crucial for controlling UPEC infection. By disrupting microtubules, α-hemolysin prevents the delivery of V-ATPase to phagosomes, thereby inhibiting acidification and enabling UPEC to survive longer within host cells. This demonstrates the critical role that vesicle movement and microtubule integrity play in UPEC infection and highlights how UPEC's manipulation of the host cell’s trafficking machinery contributes to its ability to evade host defenses and sustain persistent infection.
Although the exact mechanism by which α-hemolysin induces microtubule disruption in host cells is not fully understood, there are reports suggesting that hemolysin may degrade paxillin, indirectly leading to the disassembly of the cytoskeletal structure [
43,
53,
54]. Paxillin is known to regulate actin cytoskeleton reorganization and plays an essential role in cell adhesion, spreading, and migration. When microtubules are disrupted, the acidification of vesicles containing UPEC is halted, causing the phagolysosome to maintain a neutral environment conducive to UPEC survival, thereby creating an optimal setting for UPEC to establish long-term persistent infections. This strategy is similar to the tactics employed by other pathogenic bacteria, such as
Salmonella and
Mycobacteria, which prevent lysosomal acidification through effector molecules that block lysosomal fusion [
55–
57].
Although the exact reason for UPEC's inability to escape from LAMP1
+ phagolysosomal vesicles remains unclear, it is hypothesized that the heterogeneous calcium concentration within the vesicles might play a role. α-hemolysin requires calcium for maintaining high activity, as its RTX domain contains 6 calcium binding sites that enable its biological activation when bound to calcium [
58]. Interestingly, the calcium concentrations differ between the host cell, vesicles, and the cytoplasm, and even between early and late endosomes [
59]. The early endosome maintains calcium levels similar to those outside the cell, allowing hemolysin secreted by UPEC to increase its membrane-lytic activity with the aid of calcium. However, as the vesicle matures into a late endosome, calcium levels decrease drastically, which may hinder the complete formation of the hemolysin active complex. Therefore, the difference in calcium concentration between Rab27b
+ vesicles and lysosomal vesicles may act as a key factor in regulating UPEC's ability to escape from vesicles, influencing the success of persistent infection.
It is widely recognized that hemolysin secretion by UPEC is primarily associated with the lysis of host cells. However, it is notable that in infections caused by Listeria monocytogenes, which secretes large amounts of hemolysin known as Listeriolysin O, host cells do not immediately undergo lytic death. Instead, these host cells harbor a cytosolic stage of L. monocytogenes for extended periods without plasma membrane rupture. While UPEC's α-hemolysin plays a critical role in causing lytic damage to the plasma membrane, the homeostasis of the host cell membrane is also a crucial determinant of when membrane lysis is initiated. Though UPEC's mechanism does not exactly mirror that of L. monocytogenes, α-hemolysin secreted by UPEC significantly impacts the persistence of infection within the host. The toxin's influence on host cell integrity and its ability to sustain persistent infections highlights the complexity of UPEC’s pathogenic strategies.
UPEC continues to secrete hemolysin while maintaining a persistent life cycle within BECs, and the signaling events triggered by this hemolysin inside host cells play a crucial role in understanding hemolysin-mediated pathogenicity. Numerous studies have investigated these signaling mechanisms [
60,
61]. Alpha-hemolysin is particularly important during UPEC's persistent infection, as it disrupts the cellular signaling pathways of urothelial cells in a highly sophisticated manner.
One of the major advantages UPEC gains upon entering host cells is that BECs not only provide a suitable environment for UPEC survival but also offer a sanctuary from innate immune cells, such as macrophages and neutrophils, which are activated during the initial stages of infection. Additionally, UPEC minimizes its exposure to antibiotics taken by UTI patients, buying time to resume its infection cycle after antibiotic treatment has ceased. During this period, UPEC uses α-hemolysin to sustain persistent infection within the cells, with the toxin playing a complex and multifaceted role in ensuring UPEC's survival and long-term persistence. α-hemolysin thus contributes not only to immune evasion and bacterial persistence but also to the complex interactions between UPEC and the host's cellular machinery, reinforcing its pivotal role in the chronicity of UTIs.
In summary, UPEC causes UTIs and possesses the ability to induce persistent infections within BECs through various strategies (
Fig. 1). UPEC initially attaches to the bladder epithelium using adhesion factors like FimH and demonstrates the ability to invade host cells. However, to establish a persistent infection, UPEC employs virulence factors, with α-hemolysin playing a key role by disrupting cellular signaling and degrading the cytoskeleton's microtubules. This creates an environment where UPEC can survive in a neutral pH, allowing it to persist within host cells for extended periods and become a major cause of recurrent UTIs.
TREATMENT STRATEGIES FOR UTIS
Antibiotics are essential in the treatment of all types of UTIs. However, with the increasing incidence of antibiotic resistance, often linked to frequent prescriptions, accurate diagnosis is crucial to avoid unnecessary antibiotic use in conditions such as interstitial cystitis, bladder pain syndrome, and asymptomatic bacteriuria. Along with the efforts of clinicians to ensure precise diagnoses and careful prescriptions, several vaccines for UTI treatment have recently been developed, and various others are currently under research. Traditionally, developed vaccines have used uropathogens as antigens, but more recent vaccines target the pathogenic mechanisms of UTIs. Examining this vaccine development process from an immunological perspective and discussing the essential elements that future vaccines should incorporate will be of significant importance.
1. Effective Mucosal Vaccines for Recurrent UTIs
Although vaccines lack the direct ability to kill pathogens like antibiotics, they play a crucial role in preventing infections by activating the body's adaptive immune response. The antibodies produced through vaccination are a key component of humoral immunity, primarily targeting surface antigens of uropathogens. A representative vaccine in this category is ExPEC9V, which is designed to recognize conjugate polysaccharides characterized by O-antigens. ExPEC9V is an improved version of Ex-PEC4V, which was shown to reduce the recurrent UTI in women in a phase 1b clinical trial [
62,
63]. ExPEC9V is currently in clinical trials (VAC52416BAC3001) involving 18,556 elderly individuals who have a history of UTIs. This randomized, double-blind, multicenter study is currently attracting significant interest regarding its results. Antibodies targeting O-antigens exert their protective effect by binding to the pathogen's surface, physically preventing the bacteria from adhering to the urothelial cells.
Previous UTI vaccines were administered via injection, which were effective in generating antigen-specific IgG, but their efficacy in protection against UPEC challenge was not sufficiently high. This is because UTIs primarily occur on the mucosal surface covering the bladder epithelium, and injectable vaccines are less effective at stimulating mucosal immunity [
64,
65]. Therefore, generating an effective mucosal adaptive immune response is crucial, requiring the production of antibodies optimized for the mucosal surfaces infected by uropathogens to ensure protective effects. To achieve this, immunoglobulin A (IgA) secreted by B cells must undergo dimerization and glycosylation, then bind to the secretory component, which allows its secretion through the epithelial cells to the mucosa. However, IgG, which is predominantly produced by injectable vaccines, does not bind to the secretory component, resulting in low concentrations at the mucosal surface, where its activity is also inhibited by various proteins. In contrast, secretory IgA (sIgA) secreted onto the mucosal surface interacts with uropathogen antigens, trapping the pathogens and preventing direct contact with the mucosal surface, providing an “immune exclusion” effect.
Additionally, pathogens that enter epithelial vesicles can be transported back into the bladder lumen via pIgR21 or eliminated through antibody-dependent cellular cytotoxicity, which destroys locally infected cells, thus protecting the bladder epithelium [
64,
66,
67]. Designing UTI vaccines that leverage sIgA represents an effective strategy for preventing UTIs in advance.
All 3 currently developed UTI vaccines—Uro-Vaxom, Uromune, and Urovac—share a common goal of enhancing mucosal immunity. These vaccines have demonstrated clinical efficacy through randomized controlled trials, with proven effectiveness particularly in recurrent cystitis [
68,
69]. Each vaccine is designed using uropathogenic bacteria as antigens. Uro-Vaxom consists of bacterial lysates from 18 strains of uropathogenic bacteria and is administered orally. Urovac contains 10 heat-inactivated uropathogenic strains, delivered via vaginal suppository, while Uromune uses 4 strains administered sublingually. Uro-Vaxom (OM-89) is known to stimulate the gut-associated lymphoid tissue to induce an adaptive immune response [
70]. In contrast, Uromune stimulates the nasopharynx-associated lymphoid tissue to trigger an adaptive immune response [
71]. Clinical studies have reported that Uro-Vaxom reduces the incidence of recurrent UTIs; however, its vaccine efficacy is debatable depending on the clinical trial, possibly due to the antigen tolerance associated with the gut [
70,
72]. On the other hand, Uromune has been reported to be effective for recurrent UTIs [
71,
73,
74]. Although these vaccines have generally been effective in reducing the risk of recurrence, some still face the challenge of requiring long-term administration to maximize their efficiency.
UTI vaccines developed so far have utilized a mixture of various pathogens as antigens. While this strategy has been effective for antiviral vaccines, bacteria have more proteins and complex glycosylation structures than viruses, making the use of whole pathogens less efficient. As a result, recent UTI vaccine research has focused on the development of subunit vaccines that leverage adaptive immunity by targeting specific antigens. These antigens are selected for their direct involvement in pathogenicity or their optimization as T-cell epitopes. Since UTI vaccines primarily target bacterial pathogens, antigens such as FimH, O-antigens, α-hemolysin, siderophores, and various outer membrane receptors are chosen as targets.
Notably, a vaccine targeting FimC and FimH of
E. coli recently demonstrated safety and immunogenicity in phase 1a-1b clinical trials, with reports showing a robust FimH-specific antibody response that lasted for 12 months. Furthermore, patients who received 4 doses exhibited a significant reduction in recurrent UTI episodes [
75]. HlyA, a key factor for UPEC in establishing persistent infections within the bladder, is considered a promising target for vaccines aimed at preventing recurrent UTIs. A vaccine that induces antibodies and cytotoxic T cells targeting HlyA could effectively inhibit UPEC's cytotoxic activity in the early stages of infection and prevent the long-term survival of UPEC within BECs.
However, a subunit vaccine targeting FimH alone may be ineffective against pathogens other than UPEC. Therefore, the potential for developing multivalent vaccines that include antigens from multiple uropathogens, such as O-antigens from different bacterial species or shared virulence factors like fimbriae or siderophores, is essential for creating a future universal UTI vaccine.
3. Vaccine Development Strategies to Prevent Recurrent UTIs Caused by Persistent Infections
While 75% of UTI patients respond effectively to antibiotic treatment, raising questions about the necessity of UTI vaccine development, the remaining 25% suffer from recurrent UTIs and are in urgent need of additional therapies to complement existing antibiotic protocols. Although sIgA may have a preventive effect, its therapeutic efficacy could be limited. This is because recurrent cystitis is often caused by uropathogens residing inside urothelial cells in the form of IBCs or QIRs, leading to persistent infections. Therefore, effective treatment for recurrent cystitis requires targeting intracellular pathogens through cellular immunity, particularly by activating CD8+ T cells. When infected urothelial cells present uropathogen antigens via major histocompatibility complex class I molecules, activated CD8+ T cells can recognize these antigens and directly eliminate the infected cells. This cell-mediated killing by CD8+ T cells is facilitated by the release of granzyme and perforin, which induce apoptosis in the infected cells. The dying cells, along with the bacteria within them, are subsequently cleared by surrounding macrophages.
The generation of CD8
+ T cells is a complex process. In addition to interleukin-2, which is essential for T-cell survival, the initial activation of CD8
+ T cells requires cytokines such as interferon (IFN)-γ and tumor necrosis factor-α secreted by CD4
+ Th1 cells. These cytokines, additionally, promote the production of bactericidal nitric oxide (NO) via inducible NO synthase in macrophages at the infection site, enhancing bacterial killing. Therefore, the activation of CD4
+ Th1 cells is necessary. However, in recurrent bladder infections, the immune response is predominantly skewed toward a Th2 response [
76]. One reason for this shift is that during recurrent bladder infections, superficial urothelial cells undergo exfoliation, and a Th2 immune response is primarily activated to aid in tissue recovery [
76–
78]. When BECs are infected with UPEC, a distinct subset of CD301b
+OX40L
+ dendritic cells in bladder mucosa is responsible for preferentially activating TH2 cells in iliac lymph node [
76,
77]. In this context, immunization using the Th1-inducing vaccine adjuvant CpG-ODN has been shown to induce a significant increase in Th1 (CD3
+CD4
+IFN-γ
+) cells in the bladder, as demonstrated in mouse models [
77]. The Th1 immune response activates macrophages and CD8
+ T cells through IFN-γ, enabling the elimination of intracellular bacteria and infected cells, making it critical for combating persistent bacterial infections. This finding underscores the critical role these immune cells play in eliminating bacteria that remain latent within BECs.
Vaccine development has limitations because of several issues. UTIs are particularly challenging due to the persistence of IBCs and QIRs, which could potentially reactivate even after vaccination. Assessing the vaccine's efficacy over extended periods would require rigorous longitudinal studies. The vaccine's efficacy may vary across different age groups, especially considering that UTIs have high incidence rates in older adults and women. The immunosenescence observed in elderly populations may reduce vaccine efficacy, necessitating age-specific dosage adjustments or booster doses to maintain immunity. Therefore, researchers are trying to develop alternative treatment methods based on UPEC’s infection mechanisms using various compounds. Pathologically, UPEC infections occur through interactions between fimbriae and uroplakin. Thus, compounds that block this interaction could be used as therapeutics to prevent bacterial adhesion to urothelial cells and promote their clearance via urine. Natural products like D-mannose and proanthocyanidins, found in cranberry extracts, have been studied for their ability to block FimH, a key component in UPEC adhesion. The American Urological Association, the European Association of Urology, and various European organizations have evaluated the use of cranberry and D-mannose for preventing and treating UTIs, finding their benefits to be minimal [
79].
Since D-mannose has relatively low affinity for FimH, a modified compound called mannoside has been developed, which binds to FimH with a 10
6-fold higher affinity. Its effectiveness has been demonstrated in animal models, and it is currently undergoing clinical trials [
80]. However, while these treatment strategies are effective in preventing UPEC attachment to urothelial cells, they have limitations in addressing bacteria that have already invaded host cells. Currently, no therapies exist that can directly control persistent UPEC infections.
Interestingly, UPEC has been shown to promote persistent infection in BECs by inducing microtubule disruption [
16]. Animal studies have indicated that using paclitaxel, an anticancer drug, to inhibit microtubule collapse can reduce persistent infections [
16]. Although paclitaxel is known to cause side effects such as rash, hives, itching, and swelling when used as a cancer therapy [
81], intravesical administration of the drug in UTI patients is expected to result in fewer side effects, making it a potentially effective treatment for persistent bladder infections.
CONCLUSIONS
Patients with rUTIs often undergo long-term antibiotic treatments, which can lead to side effects such as toxicity in major organs and the development of antibiotic resistance. Therefore, to effectively prevent and treat recurrent UTIs, it is essential to understand the mechanisms of persistent infections and develop new therapeutic approaches. By studying the pathological mechanisms of hostpathogen interactions, we can systematically identify the pathogenic factors that contribute to persistent infections and develop targeted drugs that could significantly reduce the incidence of recurrent cystitis. Moreover, vaccine strategies that not only induce the production of neutralizing antibodies but also activate CD8+ T cells could be highly effective in addressing intracellular persistent infections. If these next-generation therapies are used in combination with antibiotics, they could create a synergistic effect, offering a new breakthrough in overcoming recurrent cystitis.
Acknowledgments
We appreciate the contributions of our colleagues who assisted in editing this manuscript.
NOTES
-
Funding/Support
This study was supported by the National Research Foundation of Korea grants (NRF-2020R1C1C1003257 and RS-2023-00221182) and the internal grant of Korea University.
-
Conflict of Interest
The authors have nothing to disclose.
-
Author Contribution
Conceptualization: HWC; Funding acquisition: HWC; Project administration: HWC; Visualization: KSA; Writing - original draft: HWC; Writing - review & editing: KSA, MN, HWC.
Fig. 1Pathomechanisms of uropathogenic Escherichia coli infection and persistence in bacterial cystitis. UPKs, uroplakins; IBC, intracellular bacterial community; PMN, polymorphonuclear neutrophil; QIR, quiescent intracellular reservoir.
REFERENCES
- 1. Yang X, Chen H, Zheng Y, Qu S, Wang H, Yi F. Disease burden and long-term trends of urinary tract infections: a worldwide report. Front Public Health 2022;10:888205.ArticlePubMedPMC
- 2. Dielubanza EJ, Schaeffer AJ. Urinary tract infections in women. Med Clin North Am 2011;95:27-41.ArticlePubMed
- 3. Timm MR, Russell SK, Hultgren SJ. Urinary tract infections: pathogenesis, host susceptibility and emerging therapeutics. Nat Rev Microbiol 2024 Sep 9 [Epub].ArticlePDF
- 4. Wagenlehner F, Wullt B, Ballarini S, Zingg D, Naber KG. Social and economic burden of recurrent urinary tract infections and quality of life: a patient web-based study (GESPRIT). Expert Rev Pharmacoecon Outcomes Res 2018;18:107-17.ArticlePubMed
- 5. Cairns C, Kang K, Santo L. National Hospital Ambulatory Medical Care Survey: 2018 emergency department summary tables [Internet]. Atlanta (GA): U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistics; [cited 2024 Oct 8]. Available from: https://www.cdc.gov/nchs/data/nhamcs/web_tables/2018-ed-web-tables-508.pdf
- 6. Hunstad DA, Justice SS. Intracellular lifestyles and immune evasion strategies of uropathogenic Escherichia coli. Annu Rev Microbiol 2010;64:203-21.ArticlePubMed
- 7. Aslam S, Albo M, Brubaker L. Recurrent urinary tract infections in adult women. JAMA 2020;323:658-9.ArticlePubMed
- 8. Brauner A, Jacobson SH, Kuhn I. Urinary Escherichia coli causing recurrent infections--a prospective follow-up of biochemical phenotypes. Clin Nephrol 1992;38:318-23.PubMed
- 9. Foxman B, Brown P. Epidemiology of urinary tract infections: transmission and risk factors, incidence, and costs. Infect Dis Clin North Am 2003;17:227-41.PubMed
- 10. Luo Y, Ma Y, Zhao Q, Wang L, Guo L, Ye L, et al. Similarity and divergence of phylogenies, antimicrobial susceptibilities, and virulence factor profiles of Escherichia coli isolates causing recurrent urinary tract infections that persist or result from reinfection. J Clin Microbiol 2012;50:4002-7.ArticlePubMedPMCPDF
- 11. Anderson GG, Palermo JJ, Schilling JD, Roth R, Heuser J, Hultgren SJ. Intracellular bacterial biofilm-like pods in urinary tract infections. Science 2003;301:105-7.ArticlePubMed
- 12. Rosen DA, Hooton TM, Stamm WE, Humphrey PA, Hultgren SJ. Detection of intracellular bacterial communities in human urinary tract infection. PLoS Med 2007;4:e329.ArticlePubMedPMC
- 13. Hannan TJ, Totsika M, Mansfield KJ, Moore KH, Schembri MA, Hultgren SJ. Host–pathogen checkpoints and population bottlenecks in persistent and intracellular uropathogenic Escherichia coli bladder infection. FEMS Microbiol Rev 2012;36:616-48.ArticlePubMedPMC
- 14. Flores-Mireles AL, Walker JN, Caparon M, Hultgren SJ. Urinary tract infections: epidemiology, mechanisms of infection and treatment options. Nature Reviews Microbiology 2015;13:269-84.ArticlePubMedPMCPDF
- 15. Whelan S, Lucey B, Finn K. Uropathogenic Escherichia coli (UPEC)-associated urinary tract infections: the molecular basis for challenges to effective treatment. Microorganisms 2023;11:2169.ArticlePubMedPMC
- 16. Naskar M, Parekh VP, Abraham MA, Alibasic Z, Kim MJ, Suk G, et al. α-Hemolysin promotes uropathogenic E. coli persistence in bladder epithelial cells via abrogating bacteria-harboring lysosome acidification. PLoS Pathog 2023;19:e1011388.ArticlePubMedPMC
- 17. Bien J, Sokolova O, Bozko P. Role of uropathogenic Escherichia coli virulence factors in development of urinary tract infection and kidney damage. Int J Nephrol 2012;2012:681473.ArticlePubMedPMCPDF
- 18. Naskar M, Choi HW. A dynamic interplay of innate immune responses during urinary tract infection. Immune Netw 2024;24:e31.ArticlePubMedPMCPDF
- 19. Zhou G, Mo WJ, Sebbel P, Min G, Neubert TA, Glockshuber R, et al. Uroplakin Ia is the urothelial receptor for uropathogenic Escherichia coli: evidence from in vitro FimH binding. J Cell Sci 2001;114:4095-103.ArticlePubMedPDF
- 20. Hooton TM, Bradley SF, Cardenas DD, Colgan R, Geerlings SE, Rice JC, et al. Diagnosis, prevention, and treatment of catheter-associated urinary tract infection in adults: 2009 International Clinical Practice Guidelines from the Infectious Diseases Society of America. Clin Infect Dis 2010;50:625-63.ArticlePubMedPDF
- 21. Stamm WE, Counts GW, Running KR, Fihn S, Turck M, Holmes KK. Diagnosis of coliform infection in acutely dysuric women. New Engl J Med 1982;307:463-8.ArticlePubMed
- 22. Connell I, Agace W, Klemm P, Schembri M, Mărild S, Svanborg C. Type 1 fimbrial expression enhances Escherichia coli virulence for the urinary tract. Proc Natl Acad Sci U S A 1996;93:9827-32.ArticlePubMedPMC
- 23. Korhonen TK, Virkola R, Holthofer H. Localization of binding sites for purified Escherichia coli P fimbriae in the human kidney. Infect Immun 1986;54:328-32.ArticlePubMedPMCPDF
- 24. Song J, Bishop BL, Li G, Grady R, Stapleton A, Abraham SN. TLR4-mediated expulsion of bacteria from infected bladder epithelial cells. Proc Natl Acad Sci U S A 2009;106:14966-71.ArticlePubMedPMC
- 25. Bishop BL, Duncan MJ, Song J, Li G, Zaas D, Abraham SN. Cyclic AMP-regulated exocytosis of Escherichia coli from infected bladder epithelial cells. Nat Med 2007;13:625-30.ArticlePubMedPDF
- 26. Mysorekar IU, Hultgren SJ. Mechanisms of uropathogenic Escherichia coli persistence and eradication from the urinary tract. Proc Natl Acad Sci U S A 2006;103:14170-5.ArticlePubMedPMC
- 27. Kerrn MB, Struve C, Blom J, Frimodt-Moller N, Krogfelt KA. Intracellular persistence of Escherichia coli in urinary bladders from mecillinam-treated mice. J Antimicrob Chemother 2005;55:383-6.ArticlePubMed
- 28. Choi N, Choi E, Cho YJ, Kim MJ, Choi HW, Lee EJ. A shared mechanism of multidrug resistance in laboratory-evolved uropathogenic Escherichia coli. Virulence 2024;15:2367648.ArticlePubMedPMC
- 29. Mulvey MA, Schilling JD, Hultgren SJ. Establishment of a persistent Escherichia coli reservoir during the acute phase of a bladder infection. Infect Immun 2001;69:4572-9.ArticlePubMedPMCPDF
- 30. De Nisco NJ, Neugent M, Mull J, Chen L, Kuprasertkul A, de Souza Santos M, et al. Direct detection of tissue-resident bacteria and chronic inflammation in the bladder wall of post-menopausal women with recurrent urinary tract infection. J Mol Biol 2019;431:4368-79.ArticlePubMedPMC
- 31. Eto DS, Sundsbak JL, Mulvey MA. Actin-gated intracellular growth and resurgence of uropathogenic Escherichia coli. Cell Microbiol 2006;8:704-17.ArticlePubMed
- 32. Schreiber HL 4th, Conover MS, Chou WC, Hibbing ME, Manson AL, Dodson KW, et al. Bacterial virulence phenotypes of Escherichia coli and host susceptibility determine risk for urinary tract infections. Sci Transl Med 2017;9:eaaf1283.ArticlePubMedPMC
- 33. Rippere-Lampe KE, O'Brien AD, Conran R, Lockman HA. Mutation of the gene encoding cytotoxic necrotizing factor type 1 (cnf(1)) attenuates the virulence of uropathogenic Escherichia coli. Infect Immun 2001;69:3954-64.ArticlePubMedPMCPDF
- 34. Mobley HL, Green DM, Trifillis AL, Johnson DE, Chippendale GR, Lockatell CV, et al. Pyelonephritogenic Escherichia coli and killing of cultured human renal proximal tubular epithelial cells: role of hemolysin in some strains. Infect Immun 1990;58:1281-9.ArticlePubMedPMCPDF
- 35. O'Hanley P, Lalonde G, Ji G. Alpha-hemolysin contributes to the pathogenicity of piliated digalactoside-binding Escherichia coli in the kidney: efficacy of an alpha-hemolysin vaccine in preventing renal injury in the BALB/c mouse model of pyelonephritis. Infect Immun 1991;59:1153-61.ArticlePubMedPMCPDF
- 36. Nagy G, Altenhoefer A, Knapp O, Maier E, Dobrindt U, Blum-Oehler G, et al. Both alpha-haemolysin determinants contribute to full virulence of uropathogenic Escherichia coli strain 536. Microbes Infect 2006;8:2006-12.PubMed
- 37. Sandberg T, Kaijser B, Lidin-Janson G, Lincoln K, Orskov F, Orskov I, et al. Virulence of Escherichia coli in relation to host factors in women with symptomatic urinary tract infection. J Clin Microbiol 1988;26:1471-6.ArticlePubMedPMCPDF
- 38. Orskov I, Svanborg Eden C, Orskov F. Aerobactin production of serotyped Escherichia coli from urinary tract infections. Med Microbiol Immunol 1988;177:9-14.PubMed
- 39. O'Hanley P, Low D, Romero I, Lark D, Vosti K, Falkow S, et al. Gal-Gal binding and hemolysin phenotypes and genotypes associated with uropathogenic Escherichia coli. N Engl J Med 1985;313:414-20.ArticlePubMed
- 40. Marrs CF, Zhang L, Tallman P, Manning SD, Somsel P, Raz P, et al. Variations in 10 putative uropathogen virulence genes among urinary, faecal and peri-urethral Escherichia coli. J Med Microbiol 2002;51:138-42.ArticlePubMed
- 41. Smith YC, Rasmussen SB, Grande KK, Conran RM, O'Brien AD. Hemolysin of uropathogenic Escherichia coli evokes extensive shedding of the uroepithelium and hemorrhage in bladder tissue within the first 24 hours after intraurethral inoculation of mice. Infect Immun 2008;76:2978-90.ArticlePubMedPMCPDF
- 42. Nagamatsu K, Hannan TJ, Guest RL, Kostakioti M, Hadjifrangiskou M, Binkley J, et al. Dysregulation of Escherichia coli alpha-hemolysin expression alters the course of acute and persistent urinary tract infection. Proc Natl Acad Sci U S A 2015;112:E871-80.PubMedPMC
- 43. Dhakal BK, Mulvey MA. The UPEC pore-forming toxin alpha-hemolysin triggers proteolysis of host proteins to disrupt cell adhesion, inflammatory, and survival pathways. Cell Host Microbe 2012;11:58-69.PubMedPMC
- 44. Ristow LC, Welch RA. Hemolysin of uropathogenic Escherichia coli: a cloak or a dagger? Biochim Biophys Acta 2016;1858:538-45.ArticlePubMed
- 45. van Meer G, Voelker DR, Feigenson GW. Membrane lipids: where they are and how they behave. Nat Rev Mol Cell Biol 2008;9:112-24.ArticlePubMedPMCPDF
- 46. Powers ME, Kim HK, Wang Y, Bubeck Wardenburg J. ADAM10 mediates vascular injury induced by Staphylococcus aureus alpha-hemolysin. J Infect Dis 2012;206:352-6.PubMedPMC
- 47. Wankel B, Ouyang J, Guo X, Hadjiolova K, Miller J, Liao Y, et al. Sequential and compartmentalized action of Rabs, SNAREs, and MAL in the apical delivery of fusiform vesicles in urothelial umbrella cells. Mol Biol Cell 2016;27:1621-34.ArticlePubMedPMC
- 48. Yates RM, Hermetter A, Russell DG. The kinetics of phagosome maturation as a function of phagosome/lysosome fusion and acquisition of hydrolytic activity. Traffic 2005;6:413-20.ArticlePubMed
- 49. Tsang AW, Oestergaard K, Myers JT, Swanson JA. Altered membrane trafficking in activated bone marrow-derived macrophages. J Leukoc Biol 2000;68:487-94.ArticlePubMedPDF
- 50. Xu T, Forgac M. Microtubules are involved in glucose-dependent dissociation of the yeast vacuolar [H+]-ATPase in vivo. J Biol Chem 2001;276:24855-61.ArticlePubMed
- 51. Sun-Wada GH, Tabata H, Kawamura N, Aoyama M, Wada Y. Direct recruitment of H+-ATPase from lysosomes for phagosomal acidification. J Cell Sci 2009;122:2504-13.ArticlePubMedPDF
- 52. Harrison RE, Bucci C, Vieira OV, Schroer TA, Grinstein S. Phagosomes fuse with late endosomes and/or lysosomes by extension of membrane protrusions along microtubules: role of Rab7 and RILP. Mol Cell Biol 2003;23:6494-506.ArticlePubMedPMCPDF
- 53. Deakin NO, Turner CE. Paxillin comes of age. J Cell Sci 2008;121:2435-44.ArticlePubMedPMCPDF
- 54. Brown MC, Perrotta JA, Turner CE. Identification of LIM3 as the principal determinant of paxillin focal adhesion localization and characterization of a novel motif on paxillin directing vinculin and focal adhesion kinase binding. J Cell Biol 1996;135:1109-23.ArticlePubMedPMCPDF
- 55. Spano S, Gao X, Hannemann S, Lara-Tejero M, Galan JE. A bacterial pathogen targets a host rab-family gtpase defense pathway with a GAP. Cell Host Microbe 2016;19:216-26.ArticlePubMedPMC
- 56. Fratti RA, Backer JM, Gruenberg J, Corvera S, Deretic V. Role of phosphatidylinositol 3-kinase and Rab5 effectors in phagosomal biogenesis and mycobacterial phagosome maturation arrest. J Cell Biol 2001;154:631-44.ArticlePubMedPMCPDF
- 57. D'Costa VM, Braun V, Landekic M, Shi R, Proteau A, McDonald L, et al. Salmonella disrupts host endocytic trafficking by SopD2-mediated inhibition of Rab7. Cell Rep 2015;12:1508-18.ArticlePubMed
- 58. Bumba L, Masin J, Macek P, Wald T, Motlova L, Bibova I, et al. Calcium-driven folding of RTX domain beta-rolls ratchets translocation of RTX proteins through type I secretion ducts. Mol Cell 2016;62:47-62.PubMed
- 59. Gerasimenko JV, Tepikin AV, Petersen OH, Gerasimenko OV. Calcium uptake via endocytosis with rapid release from acidifying endosomes. Curr Biol 1998;8:1335-8.ArticlePubMed
- 60. Nicaud JM, Mackman N, Gray L, Holland IB. Regulation of haemolysin synthesis in E. coli determined by HLY genes of human origin. Mol Gen Genet 1985;199:111-6.ArticlePubMedPDF
- 61. Chen S, Yang D, Wen Y, Jiang Z, Zhang L, Jiang J, et al. Dys-regulated hemolysin liberates bacterial outer membrane vesicles for cytosolic lipopolysaccharide sensing. PLoS Pathog 2018;14:e1007240.ArticlePubMedPMC
- 62. Huttner A, Hatz C, van den Dobbelsteen G, Abbanat D, Hornacek A, Frolich R, et al. Safety, immunogenicity, and preliminary clinical efficacy of a vaccine against extraintestinal pathogenic Escherichia coli in women with a history of recurrent urinary tract infection: a randomised, single-blind, placebo-controlled phase 1b trial. Lancet Infect Dis 2017;17:528-37.ArticlePubMed
- 63. Frenck RW Jr, Ervin J, Chu L, Abbanat D, Spiessens B, Go O, et al. Safety and immunogenicity of a vaccine for extra-intestinal pathogenic Escherichia coli (ESTELLA): a phase 2 randomised controlled trial. Lancet Infect Dis 2019;19:631-40.ArticlePubMed
- 64. Neutra MR, Kozlowski PA. Mucosal vaccines: the promise and the challenge. Nat Rev Immunol 2006;6:148-58.ArticlePubMedPDF
- 65. Lamm ME. Interaction of antigens and antibodies at mucosal surfaces. Annu Rev Microbiol 1997;51:311-40.ArticlePubMed
- 66. van Egmond M, Damen CA, van Spriel AB, Vidarsson G, van Garderen E, van de Winkel JG. IgA and the IgA Fc receptor. Trends Immunol 2001;22:205-11.ArticlePubMed
- 67. Black KP, Cummins JE Jr, Jackson S. Serum and secretory IgA from HIV-infected individuals mediate antibody-dependent cellular cytotoxicity. Clin Immunol Immunopathol 1996;81:182-90.ArticlePubMed
- 68. Loubet P, Ranfaing J, Dinh A, Dunyach-Remy C, Bernard L, Bruyere F, et al. Alternative therapeutic options to antibiotics for the treatment of urinary tract infections. Front Microbiol 2020;11:1509.ArticlePubMedPMC
- 69. Aziminia N, Hadjipavlou M, Philippou Y, Pandian SS, Malde S, Hammadeh MY. Vaccines for the prevention of recurrent urinary tract infections: a systematic review. BJU Int 2019;123:753-68.ArticlePubMedPDF
- 70. Brodie A, El-Taji O, Jour I, Foley C, Hanbury D. A retrospective study of immunotherapy treatment with Uro-Vaxom (OM-89(R)) for prophylaxis of recurrent urinary tract infections. Curr Urol 2020;14:130-4.PubMedPMC
- 71. Nickel JC, Doiron RC. An effective sublingual vaccine, mv140, safely reduces risk of recurrent urinary tract infection in women. Pathogens 2023;12:359.ArticlePubMedPMC
- 72. Cruz F, Dambros M, Naber KG, Bauer HW, Cozma G. Recurrent urinary tract infections: Uro-Vaxom® a new alternative. Eur Urol Suppl 2009;8:762-8.Article
- 73. Kovacic J, Canagasingham A, Zhong W, Lockhart K, Dhar A, Shepherd A, et al. Evaluation of MV140 in preventing recurrent urinary tract infections: a multicentre double-blind randomized controlled trial protocol. BJU Int 2024;133:Suppl 4. 37-43.PubMed
- 74. Lorenzo-Gomez MF, Foley S, Nickel JC, Garcia-Cenador MB, Padilla-Fernandez BY, Gonzalez-Casado I, et al. Sublingual MV140 for prevention of recurrent urinary tract infections. NEJM Evid 2022;1:EVIDx2200081.PubMed
- 75. Eldridge GR, Hughey H, Rosenberger L, Martin SM, Shapiro AM, D'Antonio E, et al. Safety and immunogenicity of an adjuvanted Escherichia coli adhesin vaccine in healthy women with and without histories of recurrent urinary tract infections: results from a first-in-human phase 1 study. Hum Vaccin Immunother 2021;17:1262-70.ArticlePubMed
- 76. Wu J, Hayes BW, Phoenix C, Macias GS, Miao Y, Choi HW, et al. A highly polarized T(H)2 bladder response to infection promotes epithelial repair at the expense of preventing new infections. Nat Immunol 2020;21:671-83.ArticlePubMedPMCPDF
- 77. Wu J, Bao C, Reinhardt RL, Abraham SN. Local induction of bladder Th1 responses to combat urinary tract infections. Proc Natl Acad Sci U S A 2021;118:e2026461118.ArticlePubMedPMC
- 78. Suk G, Kwon DH, Roers A, Abraham SN, Choi HW. Stabilization of activated mast cells by ORAI1 inhibitor suppresses peanut-induced anaphylaxis and acute diarrhea. Pharmacol Res 2023;196:106887.ArticlePubMed
- 79. Konesan J, Liu L, Mansfield KJ. The clinical trial outcomes of cranberry, D-Mannose and NSAIDs in the prevention or management of uncomplicated urinary tract infections in women: a systematic review. Pathogens 2022;11:1471.ArticlePubMedPMC
- 80. Mydock-McGrane L, Cusumano Z, Han Z, Binkley J, Kostakioti M, Hannan T, et al. Antivirulence C-Mannosides as antibiotic-sparing, oral therapeutics for urinary tract infections. J Med Chem 2016;59:9390-408.ArticlePubMedPMCPDF
- 81. Sibaud V, Leboeuf NR, Roche H, Belum VR, Gladieff L, Deslandres M, et al. Dermatological adverse events with taxane chemotherapy. Eur J Dermatol 2016;26:427-43.ArticlePubMedPMC
 , Manisha Naskar
, Manisha Naskar , Hae Woong Choi
, Hae Woong Choi 


 KAUTII
KAUTII
 ePub Link
ePub Link Cite
Cite