Abstract
Anogenital wart caused by human papillomavirus (HPV) is the most common sexually transmitted infection. High-risk strains, such as types 16 and 18, cause penile cancer in men, cervical and vulvar cancers in women, and head and neck cancers and anal cancer in both sexes. Since these malignant tumors can be prevented through vaccination, the importance of vaccination is emphasized. However, because HPV is known to cause cervical cancer, vaccination is only being administered to women. Some countries vaccinate men as well, but in South Korea, only girls are included in the National Immunization Program. However, screening for HPV in men is not possible, and the virus causes various malignant tumors, with a sharp increase in head and neck cancers, as well as a surge in genital warts in the country. In addition, HPV worsens sperm quality. Moreover, the need for vaccines is increasing as the known methods for preventing HPV-related diseases in men are decreasing and the disease burden is increasing. As cost-effectiveness studies have shown that the cost-effectiveness of vaccination is lower for men than for women, it is unlikely that male vaccination will be included in national immunization programs. Many countries overseas, especially a very small number of OECD (Organization for Economic Cooperation and Development) countries including South Korea, are implementing mandatory vaccination for women. Vaccinating men and women, would be cost-effective and efficient in achieving herd immunity. In addition to herd immunity, the inclusion of male vaccination in the National Immunization Program is imperative given the rapidly increasing incidence of diseases in men.
-
Keywords: Human papillomavirus, Vaccination, Uterine cervical neoplasms, Oropharyngeal neoplasms, Condyloma acuminata
HIGHLIGHTS
Men are also affected by various human papillomavirus (HPV)-related diseases, and the incidence of HPV-associated malignancies in men is on the rise. However, there are no appropriate screening tests available for men. Including men in the HPV vaccination program is also an efficient way to achieve herd immunity. HPV infection is known to reduce sperm quality, and the rate of circumcision, which has been suggested to help prevent HPV infection, is decreasing. Just as HPV-related diseases are prevented in women through vaccination, it is essential to include men in the national vaccination program as well. Additionally, catch-up vaccination should be implemented by expanding the age range for vaccination eligibility.
INTRODUCTION
Human papillomavirus (HPV) can appear in anyone who is sexually active. In most cases, people become infected but show no symptoms or develop any diseases. The virus often remains in the body but naturally disappears in about 90% of cases [
1]. However, in some instances where the infection persists in the body, it can penetrate tissues and lead to malignant tumors [
2]. It is known that many of the malignant tumors caused by HPV can be largely prevented through vaccination. However, achieving herd immunity against HPV involves many challenges, such as reaching high vaccination rates and ensuring vaccination for both men and women without distinction [
3–
6]. Many countries around the world, especially OECD (Organization for Economic Cooperation and Development) countries, have implemented HPV vaccination as part of their national immunization programs for men as well [
7–
9]. However, in South Korea, the National Immunization Program currently targets only girls and some low-income women. In order to achieve herd immunity, a certain level of vaccination coverage must be reached. This discussion seeks to explore, from various perspectives, why HPV vaccination is also necessary for men.
WHAT IS HPV?
HPV belongs to the family Papillomaviridae and is a nonenveloped, double-stranded DNA virus [
10]. There are over 200 species of HPV, and about 30 to 40 types cause diseases in the genital and anal regions [
11]. HPV mainly invades epithelial cells and mucous layers, has a long incubation period, and can remain in the body for a long time. In most cases, it is confined to the infected area and remains asymptomatic, often disappearing naturally after about 18 months without special treatment [
12]. However, in some cases, the virus persists in the body or penetrates deeper into tissues, causing diseases [
13]. HPV types are classified into high-risk and low-risk groups, with about 20 types categorized as high-risk, causing malignant tumors, including types 16 and 18 (
Table 1). Low-risk types, such as types 6 and 11, cause diseases like genital warts. When classified by gender, it causes head and neck cancer, anal cancer, and genital warts in both men and women, cervical cancer and vulvar cancer in women, and penile cancer in men.
Initially, attention was drawn due to its close association with cervical cancer in women, and the concept of being a preventable cancer through vaccination led to a Nobel Prize and garnered global interest in terms of women's health rights, with many countries actively engaging in efforts [
14]. In 2018, the World Health Organization made the following statement, urging the world to participate in combating HPV-related diseases and emphasizing the importance of prevention through vaccination.
In 2018, it was reported that approximately 30 million cases of genital warts occurred worldwide, along with 530,000 cases of cervical cancer, 20,000 cases of female genital tumors, 38,000 cases of head and neck cancer, 13,000 cases of penile cancer, and 35,000 cases of anal cancer [
15–
19]. These conditions are transmitted through sexual activity and primarily cause cervical cancer in women. Currently, in most countries, vaccination is provided for women under the assumption that the health risks for women are significant. However, many countries are also vaccinating men, as they have determined that vaccinating men contributes to disease eradication, achieving herd immunity, and that gender-neutral vaccination is cost-effective.
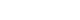
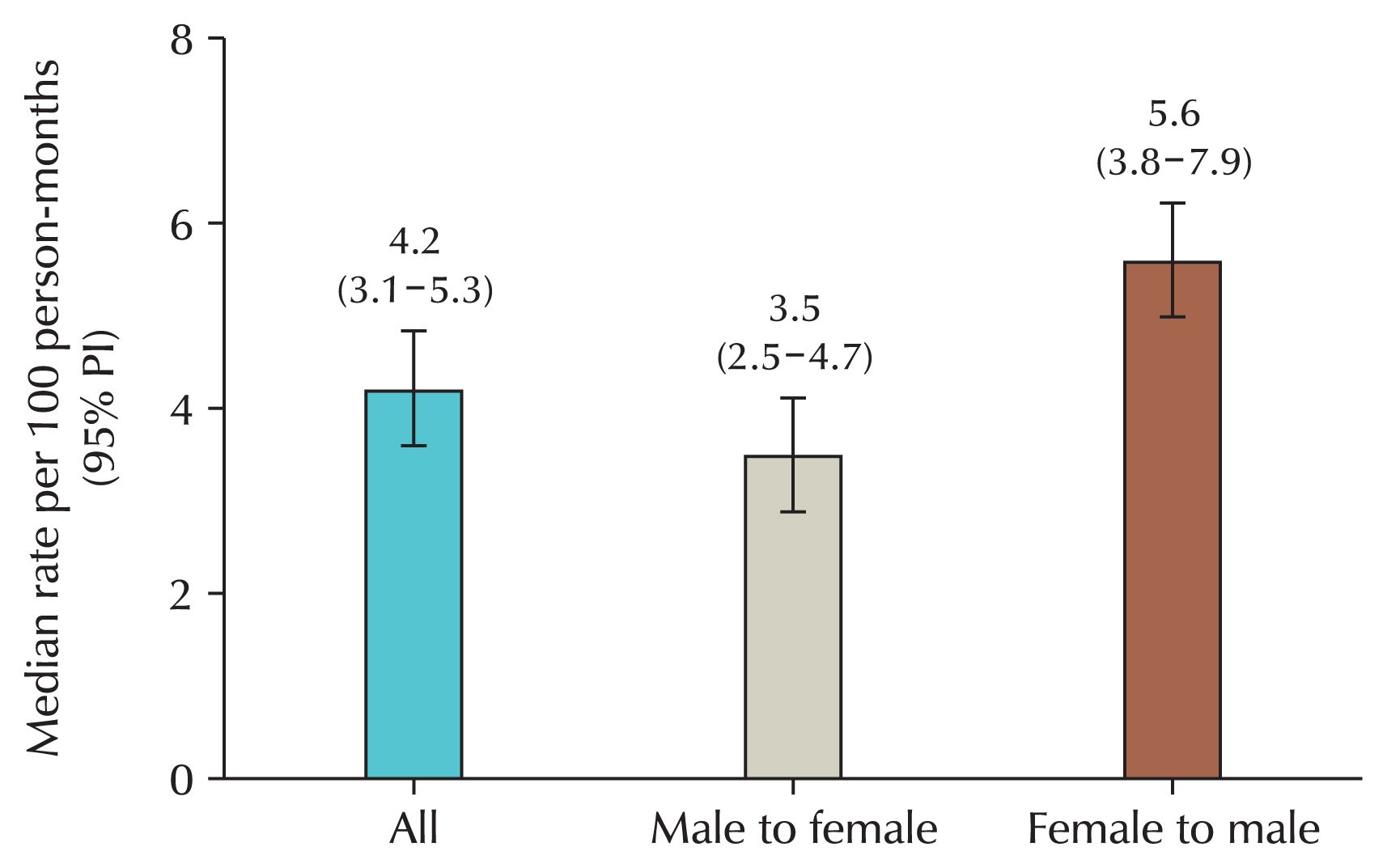
When looking at the rate of transmission through sexual intercourse, the rate from women to men is confirmed to be higher than from men to women [
20]. Although this is due to differences in anatomical structure and the possibility of the virus remaining in the human body, the transmission rate from men to women is also not small, so vaccination only for women is limited (
Fig. 1). After all, women are transmitted from men.
CURRENT STATUS OF HPV VACCINATION IN KOREA
In 2006, the quadrivalent vaccine Gardasil was approved by the U.S. Food and Drug Administration (FDA), and in 2007, the bivalent HPV vaccine was launched by GSK (GlaxoSmithKline, London, UK). In Korea, the bivalent vaccine was approved in 2007 and the quadrivalent vaccine in 2008. Thanks to its clear preventive effects against cervical cancer, HPV vaccination was introduced into the National Immunization Program for girls aged 12 in 2016. However, as of January 2023, vaccination for boys has not yet begun. The nonavalent vaccine, which includes five additional types, was launched in 2016. In 2022, the vaccination age for women was expanded to include ages 13 to 17, and low-income groups were supported until age 26. The U.S. FDA also approved the vaccine for head and neck cancer in 2022, and approval in Korea is expected soon. The indications for the catchup vaccine for women have been expanded to 45 years of age. Currently, the HPV vaccines in Korea that are indicated for males are 4-valent and 9-valent. However, men are not receiving national support regardless of age. Recently, in terms of youth health and women's health, the government and the National Assembly have legislated to include men and to vaccinate adolescents regardless of gender by the age of 12 or 17, and interest in including male vaccination is increasing as the 20th President's strategy includes male vaccination. In addition, the vaccination age has been extended to 45 years for females, while it is 26 years for males. The indications for females have been expanded to the age at which vaccination is effective, but males, who are included in the national vaccination in other countries such as Australia, have not been approved for national vaccination or have their vaccination age expanded due to insufficient research on male diseases related to HPV in Korea.
WHY IS HPV VACCINATION NECESSARY FOR MEN?
1. Men Can Also Contract Diseases Caused by HPV
Despite initially being known as the “cervical cancer virus” and “cervical cancer vaccine,” HPV also affects men. The misconception that HPV only concerns women has hindered awareness of its impact on men. HPV infection also causes many diseases in men, and some diseases occur only in men or occur more often in men. HPV-related diseases in men include penile cancer, anal cancer, and head and neck cancer, anogenital warts (
Table 2).
Anogenital warts are one of the most common sexually transmitted infections and the most prevalent of HPV-related diseases. Genital warts are another common condition in men. Although penile cancer is relatively rare, it is associated with HPV, as are anal and head and neck cancers. In a study of HPV-related tumors, when HPV DNA was detected in approximately 16,000 malignant tumor specimens, anal cancer and cervical cancer showed the highest correlation at 88.3% and 84.8%, while penile cancer and head and neck cancer also showed correlation at 33% and 25%, respectively [
21,
22].
The prevalence of HPV-related disease is rapidly increasing in Korea, particularly among men. In a study using data from the Health Insurance Review and Assessment Service (HIRA) from 2007 to 2015, while the prevalence among women showed a decreasing trend, it continued to increase steadily among men. Additionally, the associated medical costs have also shown a rising trend [
23]. Subsequently, extending the period to 2018 and segmenting the data by age, the age-specific prevalence, medical costs, and the total number of patients continued to show an increasing trend [
24]. The number of patients increased by approximately 350% from 2007 to 2018. Even taking into account the shortcomings of the diagnostic-based data, we can see that the treatment-related portion is also increasing rapidly in men, and that anogenital warts are increasing rapidly, centering on the increase in men overall [
25].
Approximately 5% of all cancers worldwide, around 730,000 cases, are related to HPV. Traditionally, cervical cancer has been the most representative tumor caused by HPV. Even today, in developing countries, cervical cancer remains the most significant HPV-related malignancy. This led to the prevalent notion that HPV is primarily a concern for women. However, HPV not only affects women but also causes malignant tumors in men, including penile cancer, head and neck cancer, and anal cancer. In particular, oropharyngeal cancer (a type of head and neck cancer) is more common in men, occurring four times more often than in women, and now exceeds cervical cancer cases [
26]. Among head and neck cancers, there are malignant tumors unrelated to smoking that are associated with HPV. A Canadian study found that men develop head and neck cancer about 2.5 times more often than women [
27]. In the United States, the incidence of these HPV-related malignancies has steadily increased among men, surpassing the incidence of cervical cancer in women [
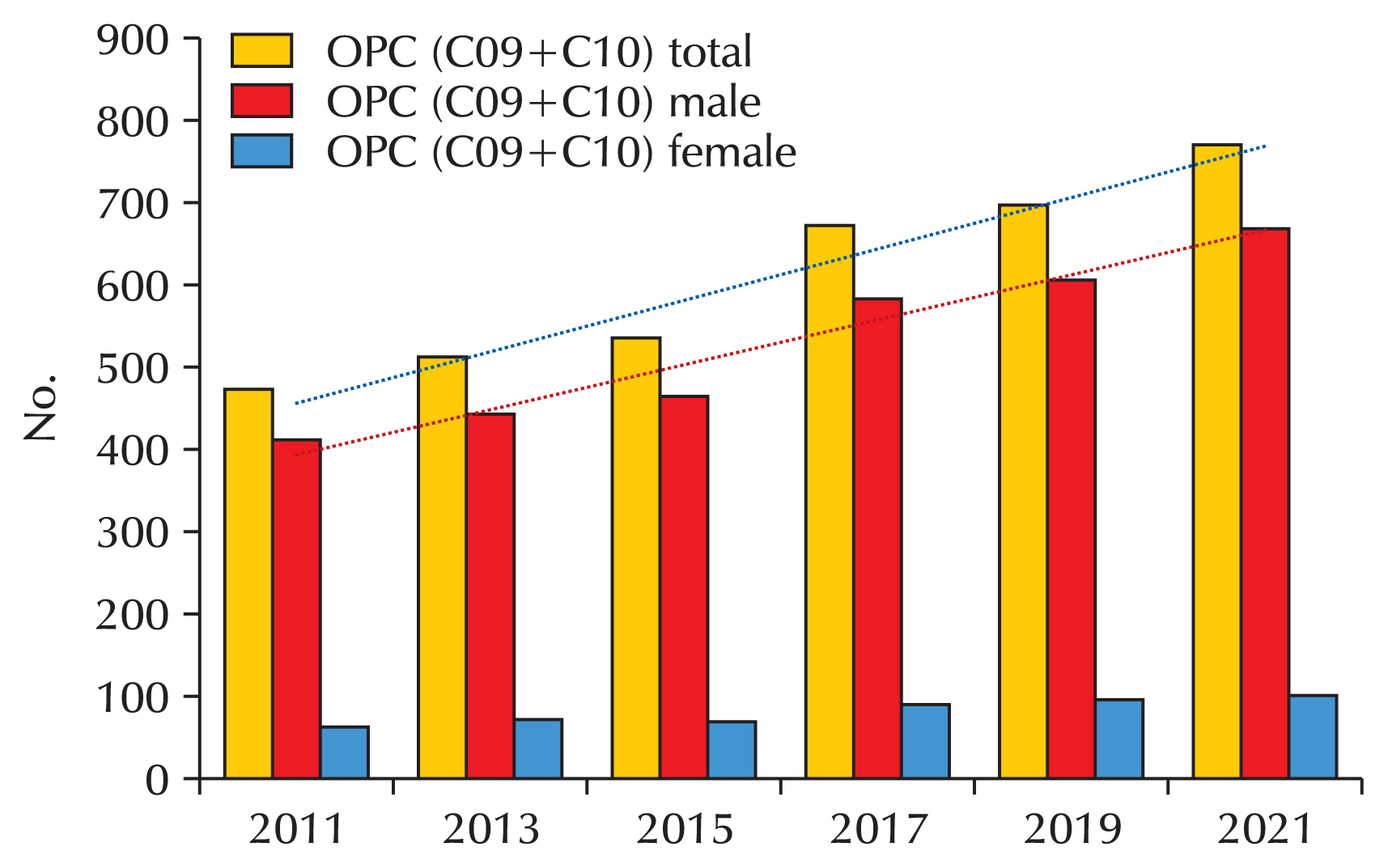
26]. Head and neck cancers are known to be closely linked to oral sex. This trend has also been observed in Korea, where population-based studies show a sharp increase in head and neck cancer cases among men [
28]. Using the big data from the HIRA, the incidence of head and neck cancer in the country has been shown to be significantly higher in men compared to women (
Fig. 2).
Penile cancer occurs mainly in people aged 50 to 70 and is closely related to HPV, of which type 16 is the most commonly found, followed by type 18 [
29,
30]. In the case of penile cancer, it is known that about 33% are related to HPV. Looking more closely, penile high-grade squamous intraepithelial neoplasia shows an HPV positivity rate of over 80%, and in the case of invasive penile cancer, it shows a correlation of 32%–35% [
22]. In Korea, the number of new cases of penile cancer increased by about 35% from 55 in 1999 to 74 in 2020. However, as can be seen from the number of patients, it is a very rare cancer. However, the number of patients showed an increasing trend [
31].
HPV infection is a state of virus retention, that is, a state of virus retention that does not cause disease, and there is a difference between the silent infection and the disease, and the virus exists in the body for a long time, about 10 years or more, and then invades the tissue and causes malignant tumors such as cervical cancer. However, in the case of men, most of the virus naturally disappears or is cleared after up to 18 months, so there is a discrepancy between the disease and the infection state. Cervical cancer can be detected early through a screening test called the Pap smear, but there are no equivalent screening methods for penile, anal, or oropharyngeal cancers. Swab tests on male genital skin have low sensitivity and are therefore unreliable [
32]. Additionally, it is not appropriate as a screening test because it appears differently depending on the location of the lesion or the shape of the sampling tool [
33]. Urine samples have a lower diagnostic rate in men than in women [
34]. Screening tests using oral samples also show similar patterns [
35]. Due to the anatomical structure of these areas, detecting the virus through smear or swab tests is difficult. However, recently, first-voided urine and oral swabs are also being considered as alternative methods [
36]. However, it does not yet reach the same level of accuracy as the screening test for women. Regular check-ups and vaccination remain the best ways to prevent these cancers in men.
A recent domestic study reported that approximately 60% of healthy adult men carry the virus [
37]. It was confirmed that even healthy men have a very high probability of having HPV as a silent infection or carrier. Therefore, they act as transmitters to women, and vaccination for prevention is necessary for transmitters as well. However, the study stated that 35% of the viruses could not be prevented by the 9-valent vaccine, requiring additional measures.
HPV vaccination started as a means of preventing cervical cancer in women, but now, about 140 countries are vaccinating women, 62 countries include men in their national vaccination programs. Many countries that implement gender-neutral vaccination are trying to increase vaccination rates to eliminate cervical cancer. Achieving full coverage of HPV-related disease requires high vaccination rates and gender-neutral vaccination [
38,
39]. However, more effort is needed to raise vaccination rates among both men and women in Korea.
In terms of vaccination rates, the gap in the HPV prevention “blind spot” for men in Korea is widening. According to the Korea Disease Control and Prevention Agency's vaccination registration database, the HPV vaccination rate for women in Korea ranges from 62.7% to 89.7%, depending on birth year. However, the highest vaccination rate among men, those born between 1983 and 1994, was only 2.1%. In contrast, Australia, which adopted gender-neutral HPV vaccination in 2013, has declared its goal to eliminate cervical cancer within 10 years. As of 2020, the HPV vaccination rate for men in Australia was nearly 83.4%. In the United Kingdom, which began supporting HPV vaccination for both men and women in 2018, the male vaccination rate was reported to be between 60% and 70% for 2022–2023.
The World Health Organization aims to vaccinate more than 90% of women [
40]. While simply increasing vaccination rates would help eradicate the disease, the real issue is cost-effectiveness. Cost-effectiveness is often considered in terms of vaccination rates by country. Korea has announced research results showing that it is more cost-effective to vaccinate only girls or to conduct catch-up vaccination for the 9-valent vaccine [
41]. In overseas studies, gender-neutral vaccination was shown to be cost-effective only in Italy for males vaccinated with the 9-valent vaccine [
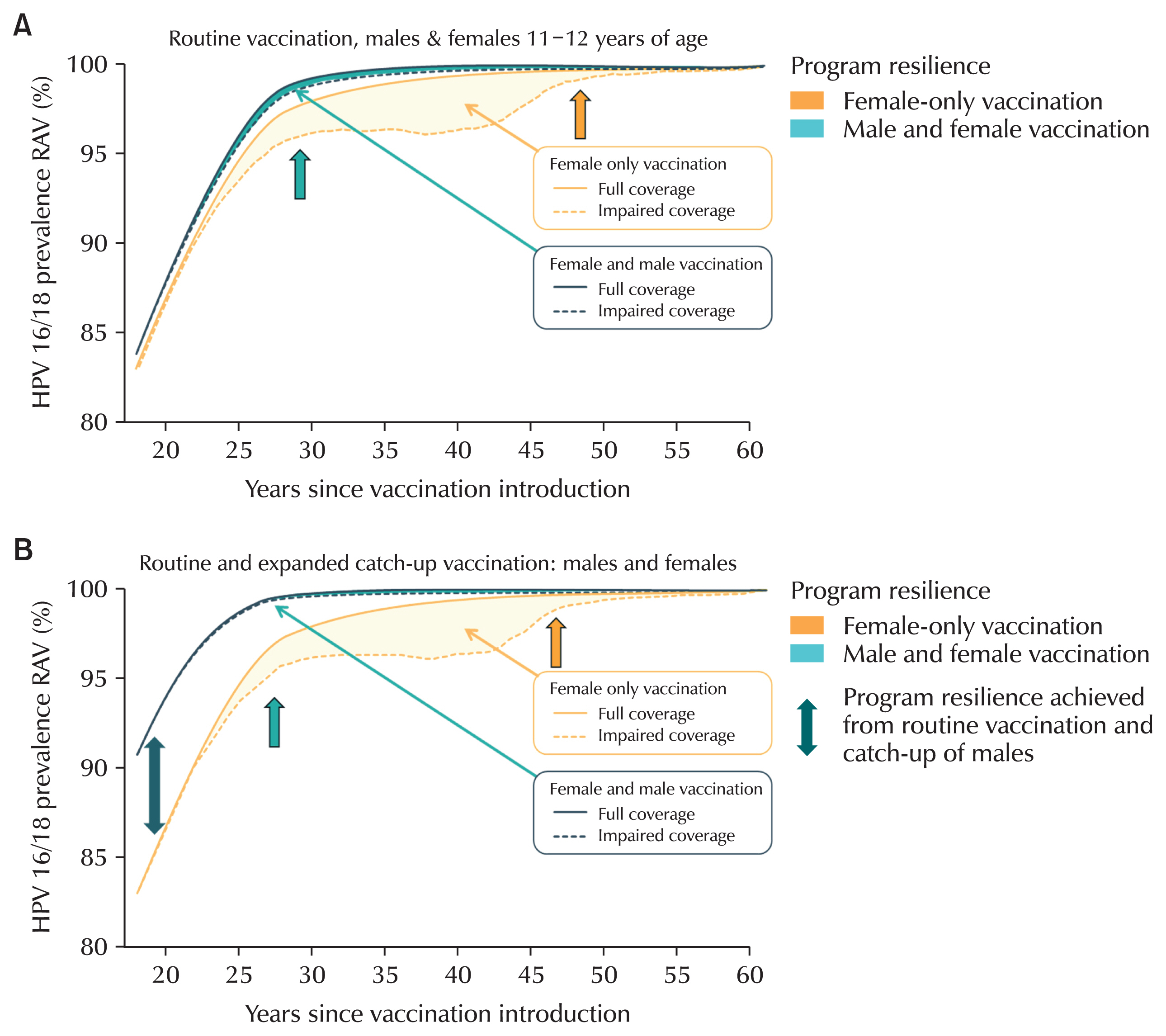
42]. However, it is said that gender-neutral vaccination can be achieved more quickly in terms of coverage of HPV disease (
Fig. 3A and B) [
38].
One of the most effective measures for preventing HPV in men is vaccination. Additionally, circumcision, specifically the ring incision procedure performed in urology, is also known to help in prevention [
43–
45]. There may be controversy about the effect of circumcision on HPV prevention and disease [
43]. In a systematic review of the literature, the annular incision has a significant effect on reducing the prevalence of HPV infection in the glans. Additionally, male circumcision can reduce the risk of several sexually transmitted diseases, improve genital hygiene, prevent malignant genital tumors, and lower the transmission rate of human immunodeficiency virus/HPV [
44]. Circumcision reduces the incidence of HPV and improves clearance (the time it takes for HPV to disappear from the body) [
45]. It is also effective in reducing the incidence of HPV in the glans, but has a minimal preventive effect on the shaft or other areas. It also reduces the incidence of infection in female partners [
46].
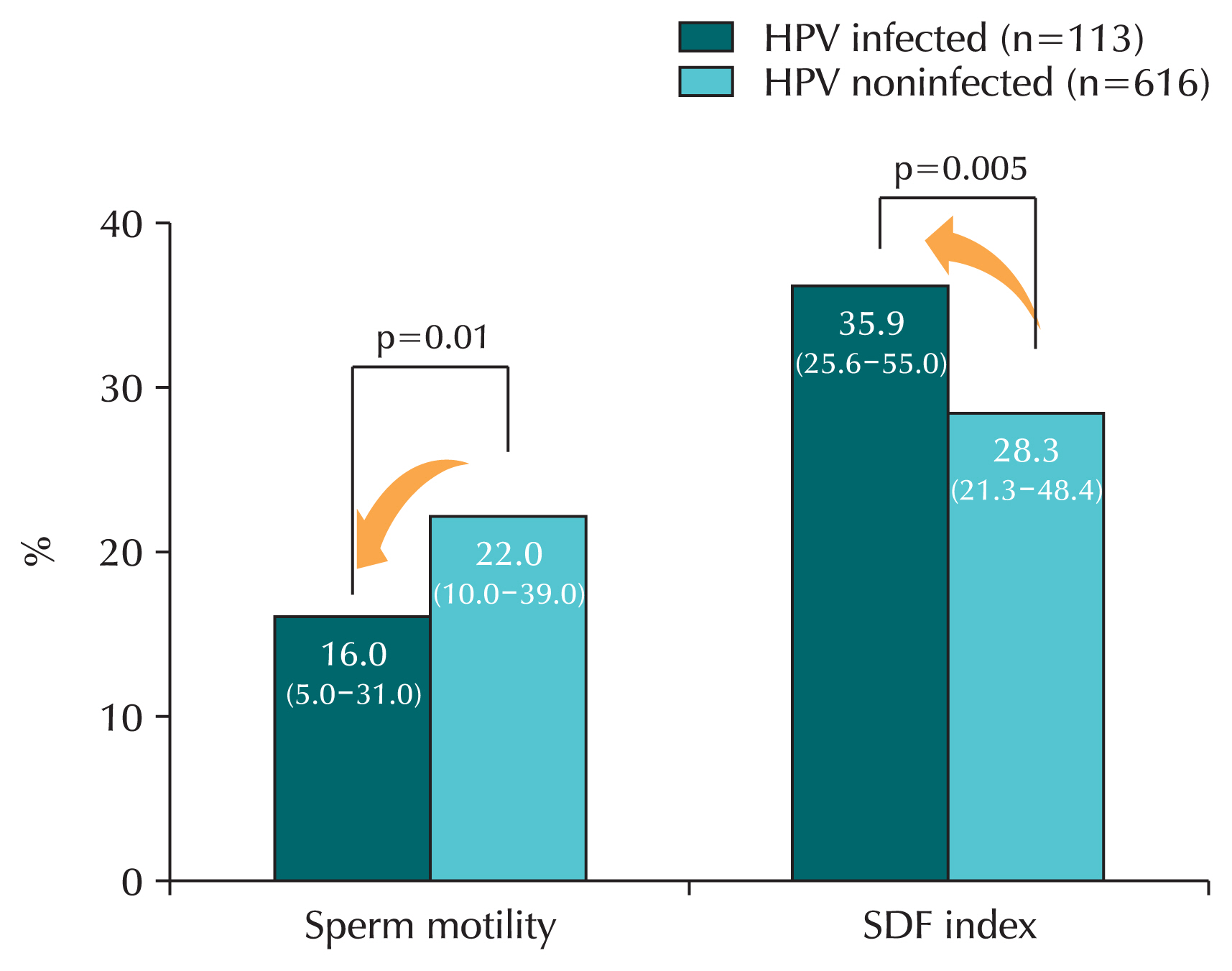
HPV infection affects sperm quality [
47–
51]. Research has shown that HPV infection in sperm decreases sperm motility and fertility, while the presence of anti-sperm antibodies increases, reducing natural pregnancy rates. When HPV infection was confirmed in semen, sperm motility was significantly reduced compared to the noninfected group [
49]. A systematic review found that sperm motility was significantly reduced in men infected with HPV compared to men who were not infected with HPV [
47]. Another study confirmed that in the HPV infection group, sperm motility was reduced and sperm DNA fragmentation index increased, which increased the degree of sperm gene destruction (
Fig. 4) [
48].
There was a study that found that the vaccine helped increase male fertility. In men with infertility, a group that received three vaccinations and a group that was observed without vaccination were compared in men who were confirmed to have HPV infection through a semen test. A study found a significant increase in normal births among vaccinated men compared to non-vaccinated men (5.5% vs. 36.7%) [
50]. However, since most men facing infertility are over the age of 30, further research in Korea is needed to expand vaccination indications beyond the current age limit of 26.
CONCLUSIONS
Diseases caused by HPV are not limited to women. Recently, HPV-related diseases such as head and neck cancer and anogenital warts have been reported to occur more frequently in men than in women. However, there are no recommended screening tests for men, and there is a conceptual disconnect between viral carriage and disease development. As a result, the burden of HPV infection in men is underestimated. While women benefit from national mandatory vaccination programs that help prevent HPV-related diseases, men do not have the same access to such preventive measures. Achieving herd immunity and preventing HPV-related diseases in men requires a greater emphasis on vaccinating males as well. Both the public and the government need to address the necessity of including men in the national vaccination program, expanding the age limit for male vaccination to improve public health.
Acknowledgments
I would like to express my gratitude to those who always provide inspiration and guidance on urinary tract infections and sexually transmitted infections. I would like to express my gratitude to Professors Seung-Ju Lee, Jin Bong Choi, Woong Bin Kim, Hong Chung, and Eun Jae Kim.
NOTES
-
Funding/Support
This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
-
Conflict of Interest
The authors have nothing to disclose.
-
Author Contribution
Conceptualization: SB; Data curation: SK; Methodology: SB, SK; Project administration: SB; Visualization: SK; Writing - original draft: SB, SK; Writing - review & editing: SB.
Fig. 1Transmission rate of human papillomavirus infection between heterosexual couples. PI, posterior interval.
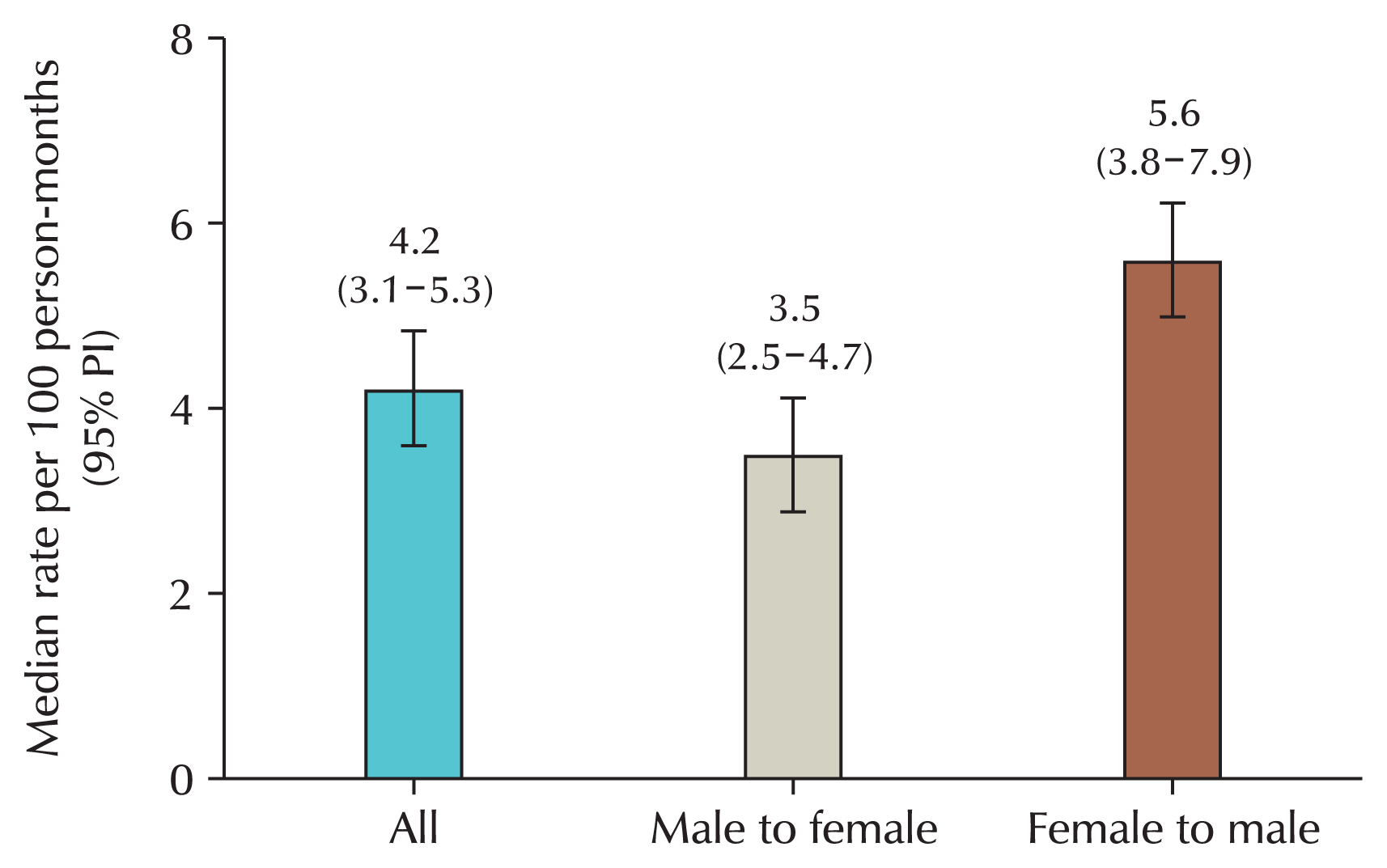
Fig. 2Incidence of oropharyngeal cancer (OPC) in Korea (Health Insurance Review and Assessment Service Big data service).
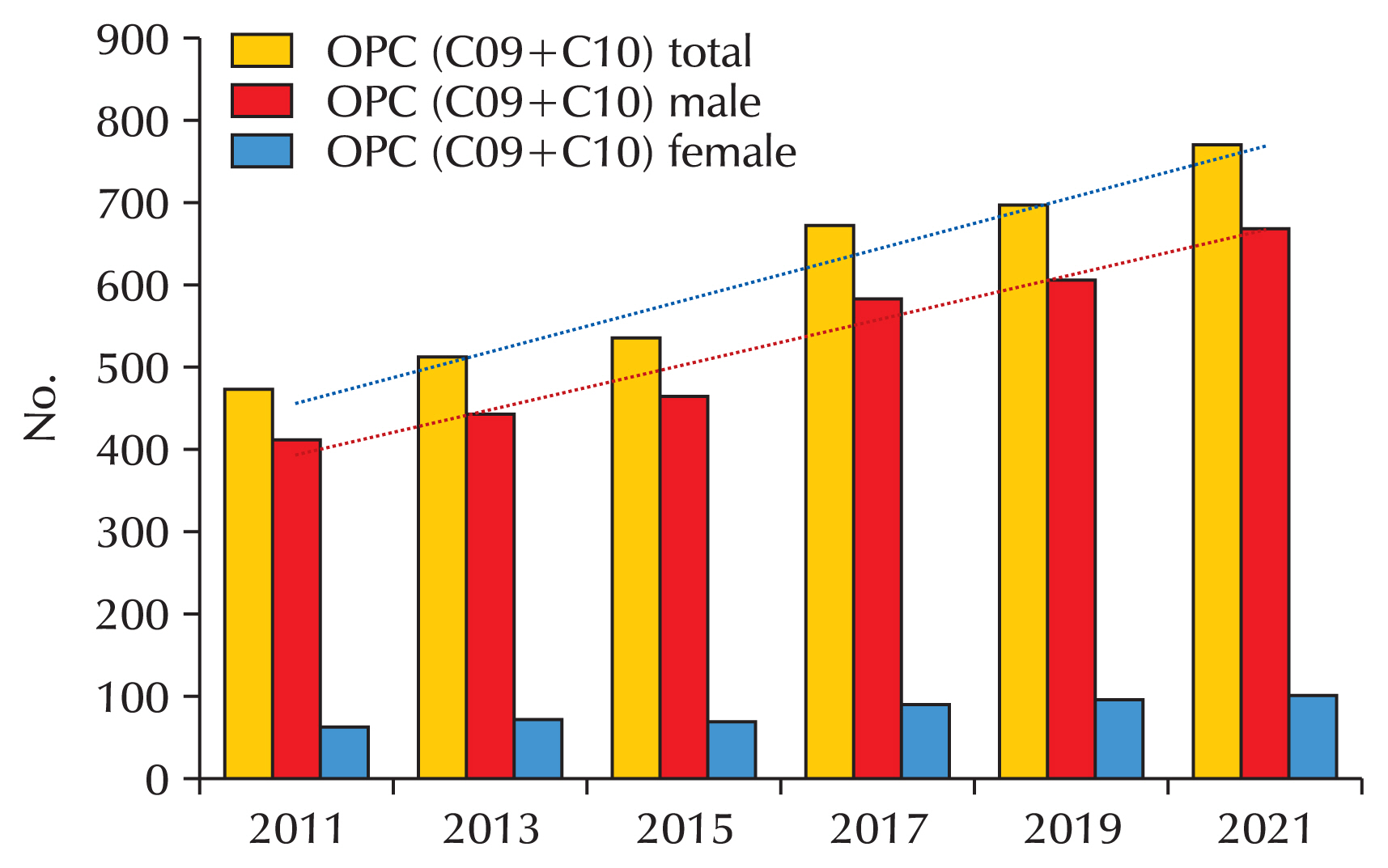
Fig. 3Comparison of gender-neutral vaccination and gender-neutral vaccination with catch-up vaccination. (A) Gender-neutral vaccination. (B) Gender-neutral vaccination with catch-up vaccination.
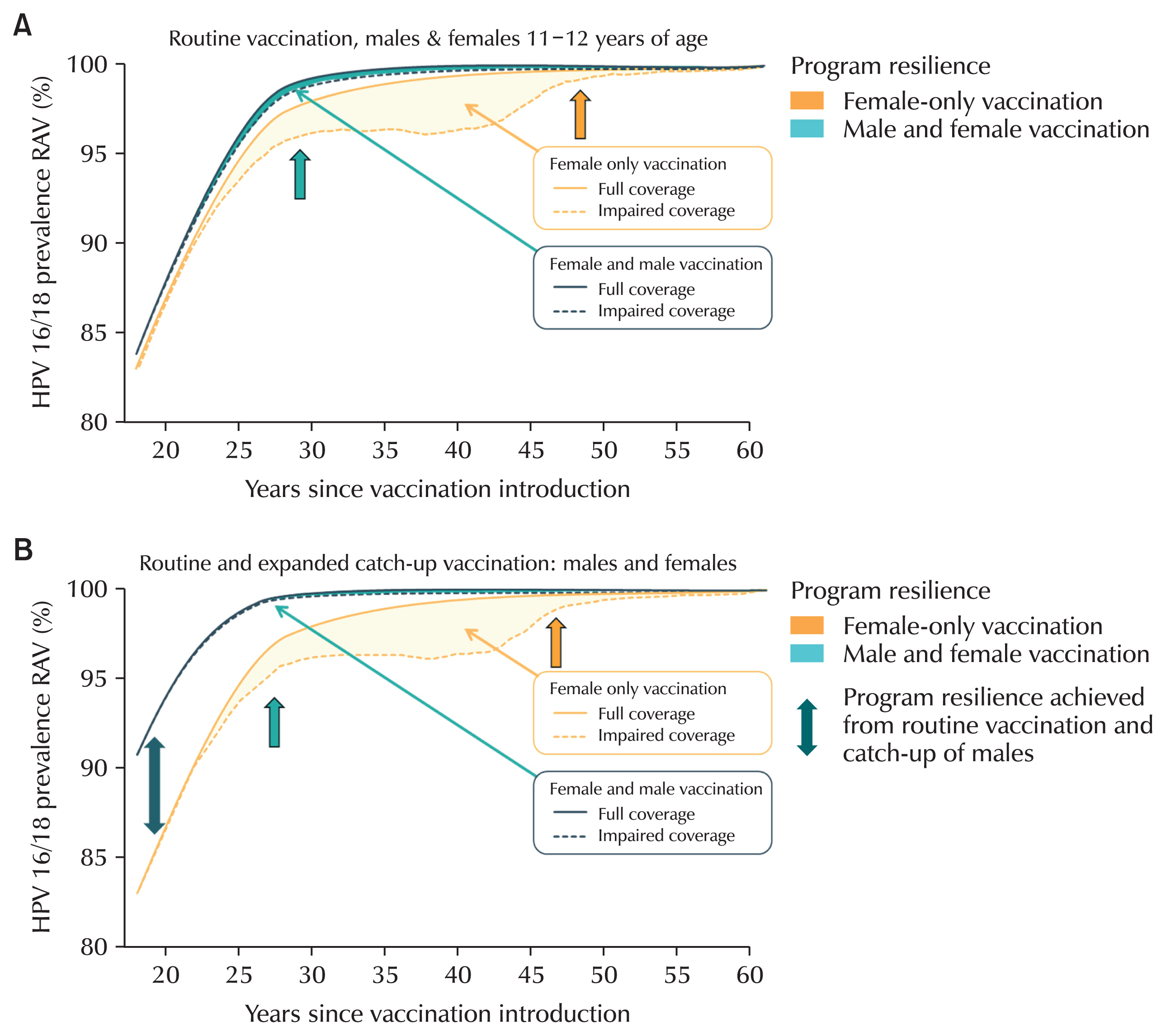
Fig. 4Sperm motility and sperm DNA fragmentation according to human papillomavirus (HPV) infection. In HPV-infected men, sperm motility was significantly decreased and sperm DNA fragmentation (SDF) was significantly increased compared to men not infected with HPV. Values are median (interquartile range).
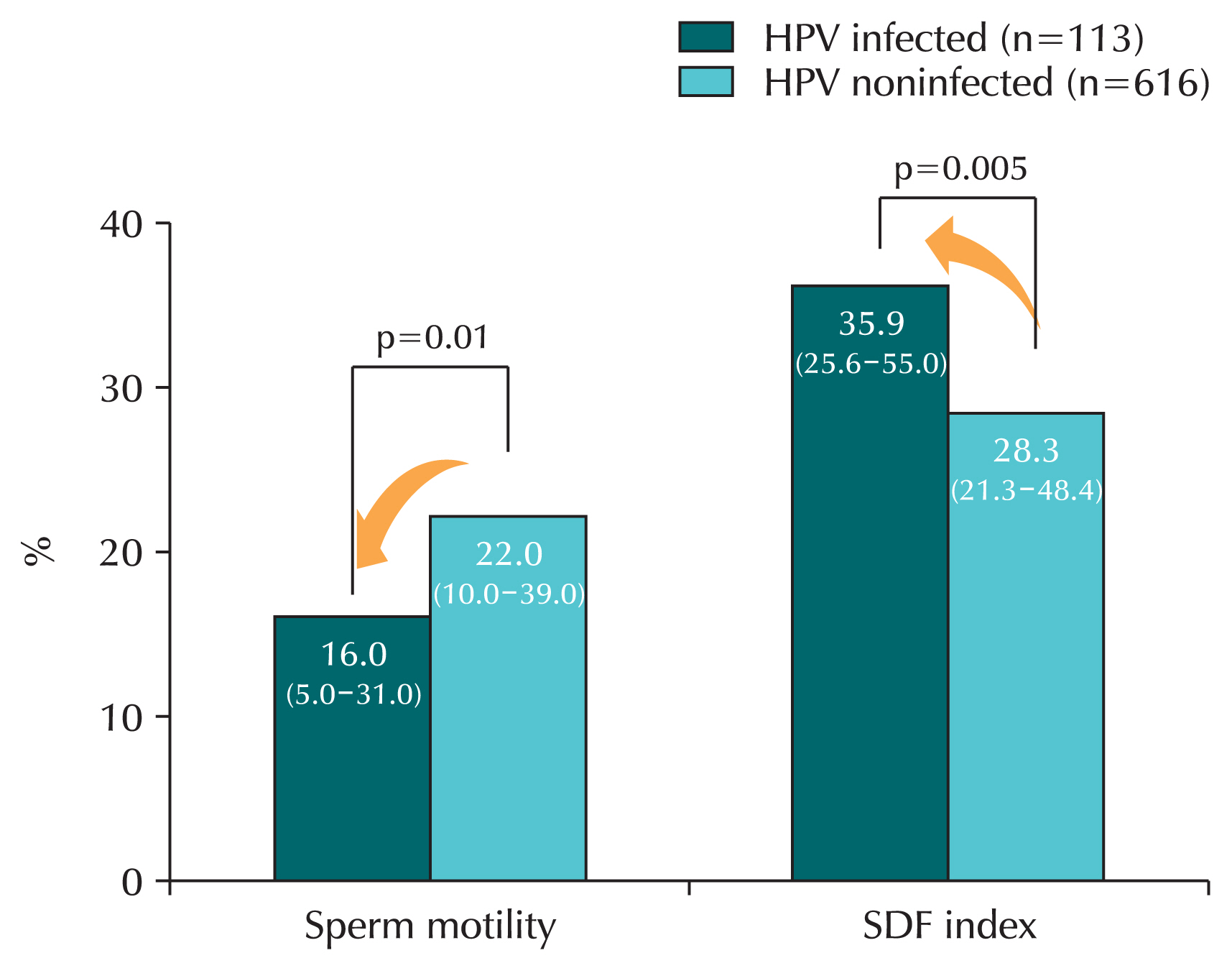
Table 1Classification of human papillomavirus viruses according to risk of malignancy
|
Group |
Subtype |
|
Low risk |
6, 11, 32, 40, 42, 43, 44, 54, 55, 61, 62, 67, 70, 72, 81 |
|
High risk |
16, 18, 31, 33, 45, 52, 58, 26, 35, 39, 51, 53, 56, 59, 66, 68, 69, 73, 82 |
Table 2various diseases depending on the various types of subtypes
|
Disease |
HPV type |
|
Common warts |
2, 7, 22 |
|
Plantar warts |
1, 2, 4, 63 |
|
Flat warts |
3, 10, 28 |
|
Anogenital warts |
6, 11, 42, 44, and others |
|
Anal dysplasia(lesions) |
6, 16, 18, 31, 53, 58 |
|
Genital cancers |
• Highest risk: 16, 18, 31, 45 |
|
• Other high-risk: 33, 35, 39, 51, 52, 56, 58, 59 |
|
• Probably high-risk: 26, 53, 66, 68, 73, 82 |
|
Epidermodysplasia verruciformis |
More than 15 types |
|
Focal epithelial hyperplasia (mouth) |
13, 32 |
|
Mouth papillomas |
6, 7, 11, 16, 32 |
|
Oropharyngeal cancer |
16 |
|
Verrucous cyst |
60 |
|
Laryngeal papillomatosis |
6, 11 |
REFERENCES
- 1. Oyouni AAA. Human papillomavirus in cancer: Infection, disease transmission, and progress in vaccines. J Infect Public Health 2023;16:626-31.ArticlePubMed
- 2. Schiffman M, Doorbar J, Wentzensen N, de Sanjosé S, Fakhry C, Monk BJ, et al. Carcinogenic human papillomavirus infection. Nat Rev Dis Primers 2016;2:16086.ArticlePubMedPDF
- 3. Williamson AL. Recent developments in human papillomavirus (HPV) vaccinology. Viruses 2023;15:1440.ArticlePubMedPMC
- 4. Malagón T, Laurie C, Franco EL. Human papillomavirus vaccination and the role of herd effects in future cancer control planning: a review. Expert Rev Vaccines 2018;17:395-409.ArticlePubMed
- 5. Simons JJM, Vida N, Westra TA, Postma MJ. Cost-effectiveness analysis of a gender-neutral human papillomavirus vaccination program in the Netherlands. Vaccine 2020;38:4687-94.ArticlePubMed
- 6. Gray P, Kann H, Pimenoff VN, Eriksson T, Luostarinen T, Vänskä S, et al. Human papillomavirus seroprevalence in pregnant women following gender-neutral and girls-only vaccination programs in Finland: A cross-sectional cohort analysis following a cluster randomized trial. PLoS Med 2021;18:e1003588.ArticlePubMedPMC
- 7. Wolff E, Elfström KM, Haugen Cange H, Larsson S, Englund H, Sparén P, et al. Cost-effectiveness of sex-neutral HPV-vaccination in Sweden, accounting for herd-immunity and sexual behaviour. Vaccine 2018;36:5160-5.ArticlePubMed
- 8. Verheijen RHM, Mahmood T, Donders G, Redman CWE, Wood P. EBCOG position statement: Gender neutral HPV vaccination for young adults. Eur J Obstet Gynecol Reprod Biol 2020;246:187-9.ArticlePubMed
- 9. Durham DP, Ndeffo-Mbah ML, Skrip LA, Jones FK, Bauch CT, Galvani AP. National- and state-level impact and cost-effectiveness of nonavalent HPV vaccination in the United States. Proc Natl Acad Sci U S A 2016;113:5107-12.ArticlePubMedPMC
- 10. Solomon IH, Milner D. Diagnostic pathology: infectious diseases-E-BOOK. Elsevier Health Sciences; 2024.
- 11. Brianti P, De Flammineis E, Mercuri SR. Review of HPV-related diseases and cancers. New Microbiol 2017;40:80-5.PubMed
- 12. Giuliano AR, Lu B, Nielson CM, Flores R, Papenfuss MR, Lee JH, et al. Age-specific prevalence, incidence, and duration of human papillomavirus infections in a cohort of 290 US men. J Infect Dis 2008;198:827-35.ArticlePubMed
- 13. Tommasino M. HPV and skin carcinogenesis. Papillomavirus Res 2019;7:129-31.ArticlePubMedPMC
- 14. Weiss RA. On viruses, discovery, and recognition. Cell 2008;135:983-6.ArticlePubMedPMC
- 15. de Martel C, Plummer M, Vignat J, Franceschi S. Worldwide burden of cancer attributable to HPV by site, country and HPV type. Int J Cancer 2017;141:664-70.ArticlePubMedPMCPDF
- 16. Ogbuji V, Gomez DM, Paster IC, Irizarry VMT, McCormick K, Dennis LK, et al. Global burden of penile cancer: a review of health disparities for a rare disease. Urology 2024;194:280-8.ArticlePubMed
- 17. Serrano B, Brotons M, Bosch FX, Bruni L. Epidemiology and burden of HPV-related disease. Best Pract Res Clin Obstet Gynaecol 2018;47:14-26.ArticlePubMed
- 18. Roman BR, Aragones A. Epidemiology and incidence of HPV-related cancers of the head and neck. J Surg Oncol 2021;124:920-2.ArticlePubMedPMCPDF
- 19. Arbyn M, Weiderpass E, Bruni L, de Sanjosé S, Saraiya M, Ferlay J, et al. Estimates of incidence and mortality of cervical cancer in 2018: a worldwide analysis. Lancet Glob Health 2020;8:e191-203.ArticlePubMed
- 20. Malagón T, MacCosham A, Burchell AN, El-Zein M, Tellier PP, Coutlée F, et al. Sex- and type-specific genital human papillomavirus transmission rates between heterosexual partners: a Bayesian Reanalysis of the HITCH Cohort. Epidemiology 2021;32:368-77.ArticlePubMedPMC
- 21. de Sanjosé S, Serrano B, Tous S, Alejo M, Lloveras B, Quirós B, et al. Burden of human papillomavirus (HPV)-related cancers attributable to HPVs 6/11/16/18/31/33/45/52 and 58. JNCI Cancer Spectr 2018;2:pky045.PubMed
- 22. de Martel C, Georges D, Bray F, Ferlay J, Clifford GM. Global burden of cancer attributable to infections in 2018: a worldwide incidence analysis. Lancet Glob Health 2020;8:e180-90.ArticlePubMed
- 23. Park YJ, Kim JM, Lee BR, Kim TH, Lee EG. Annual prevalence and economic burden of genital warts in Korea: Health Insurance Review and Assessment (HIRA) service data from 2007 to 2015. Epidemiol Infect 2018;146:177-86.ArticlePubMedPMC
- 24. Chung H, Choi JB, Kim S, Lee SJ, Bae S. Prevalence and economic burden of male anogenital wart in Korea: A population-based big data analysis from 2007 to 2018. Investig Clin Urol 2024;65:579-86.ArticlePubMedPMCPDF
- 25. Kim EJ, Lee JC, Lyu DH, Choi U, Choi JB, Kim KS, et al. Trends of genital wart in Korea according to treatment method classification: Big data analysis of health care in 2010–2019. Investig Clin Urol 2023;64:56-65.ArticlePubMedPMCPDF
- 26. Van Dyne EA, Henley SJ, Saraiya M, Thomas CC, Markowitz LE, Benard VB. Trends in human papillomavirus-associated cancers - United States, 1999–2015. MMWR Morb Mortal Wkly Rep 2018;67:918-24.ArticlePubMedPMC
- 27. Brenner DR, Gillis J, Demers AA, Ellison LF, Billette JM, Zhang SX, et al. Projected estimates of cancer in Canada in 2024. CMAJ 2024;196:E615-23.ArticlePubMedPMC
- 28. Park JO, Nam IC, Kim CS, Park SJ, Lee DH, Kim HB, et al. Sex differences in the prevalence of head and neck cancers: a 10-year follow-up study of 10 million healthy people. Cancers (Basel) 2022;14:2521.ArticlePubMedPMC
- 29. Iorga L, Dragos Marcu R, Cristina Diaconu C, Maria Alexandra Stanescu A, Pantea Stoian A, Liviu Dorel Mischianu D, et al. Penile carcinoma and HPV infection (Review). Exp Ther Med 2020;20:91-6.ArticlePubMedPMC
- 30. Mannam G, Miller JW, Johnson JS, Gullapalli K, Fazili A, Spiess PE, et al. HPV and penile cancer: epidemiology, risk factors, and clinical insights. Pathogens 2024;13:809.ArticlePubMedPMC
- 31. Cho S, Park WJ. Twenty-two-year incidence trend of urological cancers in the Republic of Korea: 1999–2020. Investig Clin Urol 2024;65:23-31.ArticlePubMedPMCPDF
- 32. Fife KH, Coplan PM, Jansen KU, DiCello AC, Brown DR, Rojas C, et al. Poor sensitivity of polymerase chain reaction assays of genital skin swabs and urine to detect HPV 6 and 11 DNA in men. Sex Transm Dis 2003;30:246-8.ArticlePubMed
- 33. Dunne EF, Nielson CM, Stone KM, Markowitz LE, Giuliano AR. Prevalence of HPV infection among men: a systematic review of the literature. J Infect Dis 2006;194:1044-57.ArticlePubMed
- 34. D'Hauwers KW, Tjalma WA. Screening for human papillomavirus: is urine useful? Indian J Cancer 2009;46:190-3.ArticlePubMed
- 35. D'Souza G, Clemens G, Troy T, Castillo RG, Struijk L, Waterboer T, et al. Evaluating the utility and prevalence of HPV biomarkers in oral rinses and serology for HPV-related oropharyngeal cancer. Cancer Prev Res (Phila) 2019;12:689-700.ArticlePubMedPMCPDF
- 36. Poljak M, Cuschieri K, Alemany L, Vorsters A. Testing for human papillomaviruses in urine, blood, and oral specimens: an update for the laboratory. J Clin Microbiol 2023;61:e0140322.ArticlePubMedPMCPDF
- 37. Ahn S, Moon D, Hwang W, Cho S, Lee H, Park H. (370) The Prevalence and Genotype Distribution of Human Papillomaviruses among Men in Korea. J Sex Med 2023;20(Supplement 1):qdad060.344; https://doi.org/10.1093/jsxmed/qdad060.344.Article
- 38. Elfström KM, Lazzarato F, Franceschi S, Dillner J, Baussano I. Human papillomavirus vaccination of boys and extended catch-up vaccination: effects on the resilience of programs. J Infect Dis 2016;213:199-205.ArticlePubMed
- 39. Lehtinen M, Apter D. Gender-neutrality, herd effect and resilient immune response for sustainable impact of HPV vaccination. Curr Opin Obstet Gynecol 2015;27:326-32.ArticlePubMed
- 40. Bruni L, Saura-Lázaro A, Montoliu A, Brotons M, Alemany L, Diallo MS, et al. HPV vaccination introduction worldwide and WHO and UNICEF estimates of national HPV immunization coverage 2010–2019. Prev Med 2021;144:106399.ArticlePubMed
- 41. Shim HW. Economic evaluation of human papillomavirus vaccination. Seoul (Korea): Seoul National University; 2021.
- 42. Linertová R, Guirado-Fuentes C, Mar Medina J, Imaz-Iglesia I, Rodríguez-Rodríguez L, Carmona-Rodríguez M. Cost-effectiveness of extending the HPV vaccination to boys: a systematic review. J Epidemiol Community Health 2021;75:910-6.ArticlePubMed
- 43. Friedman B, Khoury J, Petersiel N, Yahalomi T, Paul M, Neuberger A. Pros and cons of circumcision: an evidence-based overview. Clin Microbiol Infect 2016;22:768-74.ArticlePubMed
- 44. Mehta KS, Marfatia YS, Jain AP, Shah DJ, Baxi DS. Male circumcision and sexually transmitted infections - an update. Indian J Sex Transm Dis AIDS 2021;42:1-6.ArticlePubMedPMC
- 45. Smith JS, Backes DM, Hudgens MG, Mei W, Chakraborty H, Rohner E, et al. Male circumcision reduces penile HPV incidence and persistence: a randomized controlled trial in Kenya. Cancer Epidemiol Biomarkers Prev 2021;30:1139-48.ArticlePubMedPMCPDF
- 46. Shapiro SB, Laurie C, El-Zein M, Franco EL. Association between male circumcision and human papillomavirus infection in males and females: a systematic review, meta-analysis, and meta-regression. Clin Microbiol Infect 2023;29:968-78.ArticlePubMed
- 47. Cao X, Wei R, Zhang X, Zhou J, Lou J, Cui Y. Impact of human papillomavirus infection in semen on sperm progressive motility in infertile men: a systematic review and meta-analysis. Reprod Biol Endocrinol 2020;18:38.ArticlePubMedPMCPDF
- 48. Boeri L, Capogrosso P, Ventimiglia E, Pederzoli F, Cazzaniga W, Chierigo F, et al. High-risk human papillomavirus in semen is associated with poor sperm progressive motility and a high sperm DNA fragmentation index in infertile men. Hum Reprod 2019;34:209-17.ArticlePubMed
- 49. Foresta C, Garolla A, Zuccarello D, Pizzol D, Moretti A, Barzon L, et al. Human papillomavirus found in sperm head of young adult males affects the progressive motility. Fertil Steril 2010;93:802-6.ArticlePubMed
- 50. Garolla A, De Toni L, Bottacin A, Valente U, De Rocco Ponce M, Di Nisio A, et al. Human papillomavirus prophylactic vaccination improves reproductive outcome in infertile patients with HPV semen infection: a retrospective study. Sci Rep 2018;8:912.ArticlePubMedPMCPDF
- 51. Lyu Z, Feng X, Li N, Zhao W, Wei L, Chen Y, et al. Human papillomavirus in semen and the risk for male infertility: a systematic review and meta-analysis. BMC Infect Dis 2017;17:714.ArticlePubMedPMCPDF
 , Sangrak Bae2
, Sangrak Bae2






 KAUTII
KAUTII
 ePub Link
ePub Link Cite
Cite