Beta-Lactamase-Mediated Antibiotic Resistance in Urinary Tract Infections: Mechanisms and Therapeutic Strategies
Article information
Abstract
Urinary tract infections (UTIs) are among the most prevalent bacterial infections globally, and are primarily caused by Escherichia and Klebsiella. The overprescription and inappropriate use of antibiotics have accelerated the emergence of multidrug-resistant bacteria. Beta-lactamases play a critical role in mediating antibiotic resistance in UTIs. These enzymes promote bacterial resistance through multiple mechanisms, including gene mutation, plasmid-mediated horizontal gene transfer, and the involvement of integrons. Comprehensive knowledge of the ways in which beta-lactamases contribute to resistance in UTIs is essential for improving treatment strategies. Advances in detection technologies, such as gene sequencing and mass spectrometry, have greatly enhanced the ability to monitor and predict bacterial resistance. Current therapeutic strategies include the application of beta-lactamase inhibitors, the development of novel antibiotics, and alternative treatments that have shown efficacy against beta-lactamase-mediated antibiotic resistance. This paper reviews the mechanisms of beta-lactamase-mediated resistance in UTIs and provides an in-depth overview of several detection methods and therapeutic approaches.
INTRODUCTION
Urinary tract infections (UTIs) represent one of the most prevalent bacterial infections globally, which impact around 150 million individuals annually [1]. Common symptoms include frequent urination, a burning sensation during urination, lower abdominal pain, and fever. Risk factors include gender, age, sexual activity, past history of infection, and asymptomatic bacteriuria treatment [2]. Escherichia coli is the predominant pathogen, responsible for approximately 70%–90% of cases [3,4].
The standard treatment for UTIs involves the use of antibiotics [5]. However, the challenge of antibiotic resistance complicates the management of these infections, often resulting in prolonged disease duration, increased complications, and treatment failure, particularly in cases of antibiotic-resistant UTIs [6,7]. Beta-lactam enzymes, produced by certain bacteria, hydrolyze beta-lactam antibiotics, rendering them ineffective [8]. A significant and complex obstacle in treating UTIs is the emergence of antibiotic-resistant bacteria, particularly those producing extended-spectrum beta-lactamases (ESBLs) and metallo-beta-lactamases (MBL) [9,10]. The rise of these resistant strains has considerably diminished the effectiveness of conventional antibiotics, posing substantial challenges to clinical treatment.
A complete understanding of the resistance mechanisms associated with beta-lactamases, the development of effective detection methods, and the formulation of rational treatment strategies is crucial for the control and management of UTIs [11,12]. This review explores the mechanisms of beta-lactamases-mediated resistance in UTIs and comprehensively summarizes and showcases advanced detection methods and treatment approaches.
CLASSIFICATION OF BETA-LACTAMASES
Beta-lactamases is an enzyme produced by drug-resistant bacteria that hydrolyzes the lactam bond in beta-lactamases antibiotics, such as penicillin and cephalosporin, leading to antibiotic resistance [11]. These enzymes exhibit considerable diversity and are typically classified based on their molecular biological structure or function. According to the Ambler classification, beta-lactamases are categorized into serinases (classes A, C, and D) and metalloenzymes (class B), based on the characteristics of their terminal amino acid sequences [13]. In the functional classification, known as the Bush classification, beta-lactamases are further divided into penicillinases, broad-spectrum enzymes, ESBLs, plasmid-mediated cephalosporinase (AmpC) and carbapenemases, based on their substrate specificities, biochemical characteristics, and susceptibility to enzyme inhibitors [14].
Next generation sequencing, often referred to as high-throughput sequencing technology, enables the simultaneous sequencing of a large number of DNA or RNA molecules. This technology is characterized by its high throughput, rapid processing speed, and cost-effectiveness. It encompasses various methodologies, including metagenomic sequencing, whole genome sequencing, and transcriptome sequencing [15]. Metagenomic sequencing allows for the extraction of all microbial DNA directly from environmental samples without the need for isolation and culture, facilitating bioinformatics analyses that can identify gene sequences encoding beta-lactamases. Whole genome sequencing accurately identifies the beta-lactamases gene present in a single microbial strain by comparing it against known databases [16]. Additionally, transcriptome sequencing provides insights into gene expression levels and regulatory networks for all transcripts within a cell, thereby enhancing our understanding of beta-lactamases gene expression under various conditions [17].
SYATUS AND MECHANISM OF ANTIBIOTIC RESISTANCE MEDIATED BY BETA-LACTAMASES
1. Status of Antibiotic Resistance Mediated by Beta-Lactamases
A multitude of research has shown the extent of the global issue of antibiotic resistance in UTIs. A substantial body of research data indicates that the prevalence of ESBL-producing strains and multidrug resistant strains has significantly increased. This rise is associated with prolonged hospitadrug-resistantgher morbidity rates, posing considerable risks and challenges to clinical treatment. In particular. The extent of the antibiotic resistance issue was highlighted by a study that examined 876,507 positive urine culture samples from 322 hospitals in the United States between 2011 and 2020. The study found a significant increase in the proportion of strains that produce ESBL and strains that are resistant to multiple drugs [18]. Similar patterns were noted in other locations, such as Jiroft City, Iran, where 52.8% of E. coli isolates were found to be ESBL-producing strains and the isolates from patients with UTIs were highly resistant to beta-lactam and fluoroquinolone antibiotics [19]. Furthermore, multidrug resistant bacteria were found in urine cultures of 226 (60.1%) adult cancer patients treated at King Hussein Cancer Center in Jordan; the majority of these strains were ESBL-producing (n=142, 62.8%) [20]. Globally, and particularly in Brazil, where fluoroquinolone resistance is rising annually, the number of Gram-negative infections (such E. coli and Klebsiella pneumoniae) that are resistant to ESBL and carbapenem is rising. This is very dangerous [21]. With the majority of ESBL-E. coli isolates displaying multidrug resistance, which is linked to lengthier hospital admissions and higher morbidity, the prevalence of community-acquired ESBL-E. coli UTIs is also rising in the pediatric population [22]. According to a different study, ESBL-produced bacteria were responsible for the majority of UTIs in a Tigray referral hospital, with hospital-acquired infections being more prevalent [23]. The urethral symbiotic E. coli of elderly residents in a Ghanaian nursing home had a high detection rate of the ESBL gene, and the majority of the isolates had multiple ESBL genes. Additionally, the high rate of resistance to third- and fourth-generation cephalosporins demonstrated multidrug resistance [24]. The study also aimed to identify and characterize the prevalence of beta-lactam antibiotic resistance genes in K. pneumoniae and E. coli in patients with UTIs from western Cameroon [9]. The incidence of bacterial urinary tract pathogens that produce ESBLs in pregnant women in northwest Ethiopia is also concerning; findings indicate that 18.2% of Enterobacteriaceae bacteria produce ESBLs [25]. Antibiotic use within the previous 90 days and a history of ESBL-producing bacteria within the previous year were found to be predictors of ESBL UTIs in a study conducted by the American Medical Center in Beirut, which revealed a 24.9% prevalence of ESBL bacteria in UTIs [26]. Infections caused by MBL are associated with a higher 30-day mortality rate; therefore, effective antibiotic treatment should be administered as early as possible [27]. These studies consistently demonstrate the severity of the antibiotic resistance problem in ESBL- and MBL-producing bacteria.
2. Mechanism of Antibiotic Resistance Mediated by Beta-Lactamases
In UTIs, the mechanism of antibiotic resistance is a complex process that encompasses multiple levels, including various adaptive changes in the bacteria themselves and gene-level transmission [28]. One of the primary mechanisms of bacterial resistance is the production of beta-lactamases. This enzyme specifically targets and hydrolyzes the beta-lactam ring in beta-lactam antibiotics, rendering them ineffective. Beta-lactamases are inactivating enzymes produced by drug-resistant bacteria, capable of catalyzing the hydrolysis of lactam bonds in common beta-lactam antibiotics, such as penicillin and cephalosporin [29]. Furthermore, the extensive variety of these enzymes complicates the mechanisms of bacterial resistance and exacerbates the challenges associated with combating antibiotic resistance [30].
The researchers did many polymerase chain reaction (PCR) tests on the beta-lactamases gene in patients with ESBL-positive UTIs. They found a new CTX-M-14-like gene. This gene has 3 missense mutations: T55A, A273P, and R277C. These mutations made E. coli more resistant to nitrofurantoin [31]. CMY-192 is a new kind of beta-lactamases. It belongs to the class A cephalosporinase family. CMY-192 breaks down ceftazidime, so ceftazidime cannot work well. Moreover, CMY-192 is resistant to avibactam. As a result, the overall antibacterial effect of ceftazidime-avibactam decreased significantly [32]. Integrons are capable of capturing foreign resistance genes and expressing them in bacteria, thereby facilitating the horizontal transfer of resistance and contributing to the development of multiantibiotic resistant bacteria [33]. As mobile genetic elements, integrons play a crucial role in the transfer of antimicrobial resistance genes between bacterial populations, enhancing their resistance to antibiotics. This makes bacteria more resistant to antibiotics. This gene-transfer process is very important for spreading the genes that cause ESBL [34]. The blaCTX-M-15 gene has different effects on the resistance of E. coli in chromosome or plasmid. The blaCTX-M-15 gene carried by the plasmid leads to increased resistance to beta-lactam antibiotics [35]. Beta-lactamases facilitate bacterial resistance through various mechanisms, including gene mutation, plasmid-mediated horizontal gene transfer, and the action of integrons (Fig. 1A).
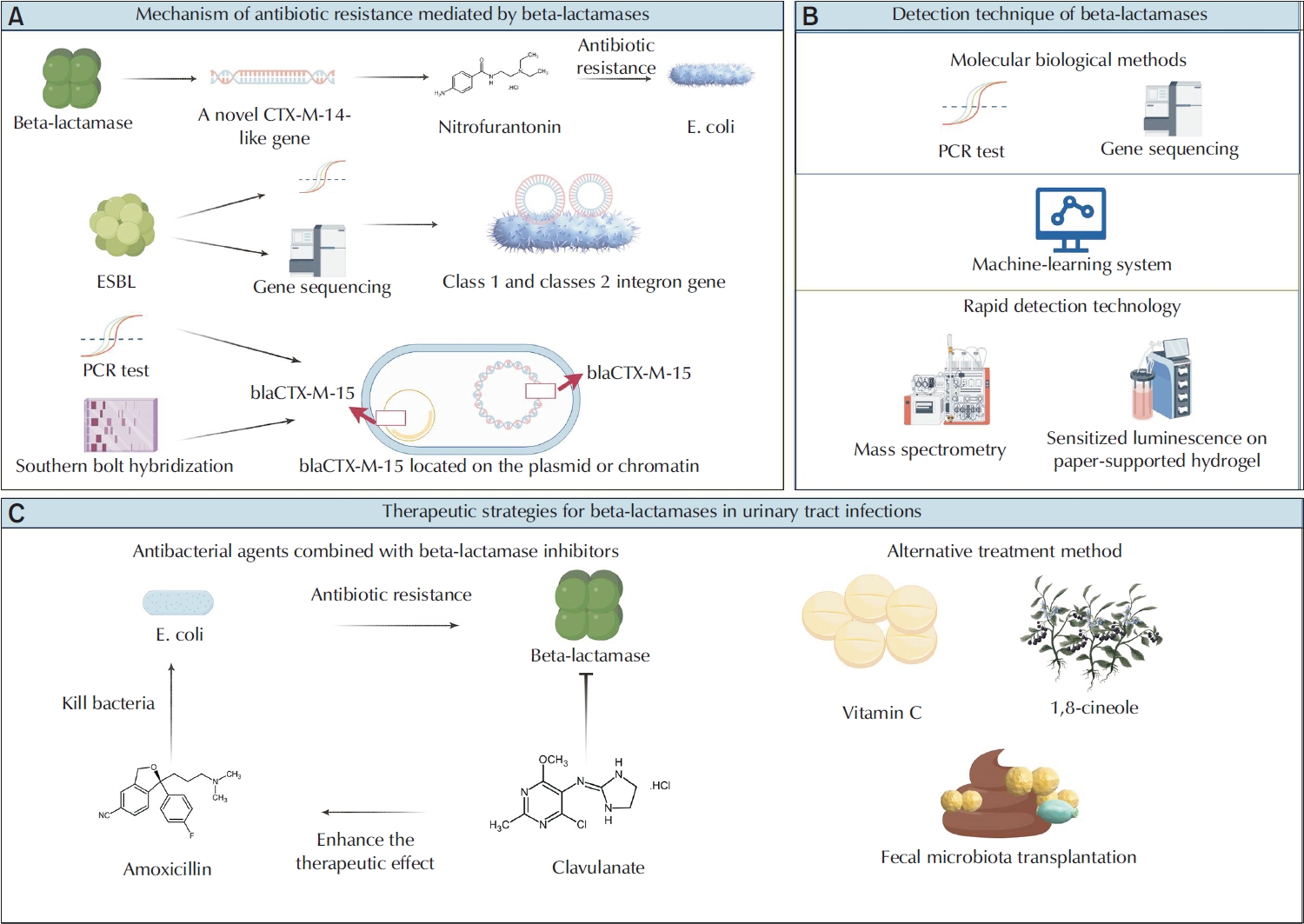
Beta-lactamases play a crucial role in the regulation of antibiotic resistance in urinary tract infections. (A) Beta-lactamases represents a crucial mechanism of antibiotic resistance. Multiplex polymerase chain reaction detections of the beta-lactamases gene in patients with ESBL-positive urinary tract infections (UTIs) have revealed a novel CTX-M-14 like gene, whose mutation significantly enhances the resistance of Escherichia coli to nitrofurantoin. As mobile genetic elements, integrons facilitate the transmission of antimicrobial resistance genes through horizontal gene transfer, particularly those encoding ESBL, thereby augmenting bacterial resistance. The impact of the blaCTX-M-15 gene on the resistance of E. coli varies depending on whether the gene is located on the chromosome or the plasmid, with the plasmid carrying this gene significantly increasing resistance to beta-lactam antibiotics. (B) Advanced detection technologies, including molecular biological methods, machine learning systems, and rapid detection technologies, have markedly improved the monitoring and prediction of antibiotic resistance. (C) Beta-lactamases inhibitors can obstruct the binding of beta-lactamases to small molecule beta-lactam antibiotics, thereby restoring the sensitivity of drug-resistant bacteria to these antibiotics and achieving an antibacterial effect. Alternative therapies, such as vitamin C,1,8-cineole, and fecal microbiota transplantation, have demonstrated significant potential in addressing UTIs and their associated drug resistance. ESBL, extended-spectrum beta-lactamase.
DETECTION TECHNIQUE OF BETA-LACTAMASES
1. New Detection Tool for Beta-Lactamases
A thorough understanding of the resistance mechanism of beta-lactamases and the development of efficient detection methods are essential for the control and treatment of UTIs. Developing new tools to detect potential targets can improve treatment and slow down the growth of antibiotic resistance. Machine learn-based methods such as Classification and Regression Trees and Random Forest can effectively predict ESBL bacteria and their multidrug resistance. Furthermore, these techniques can identify key characteristics closely related to the development of ESBL, including patient age and antibiotic usage [36]. By creating a particular substrate cephalosporin-conjugated sensitizer that releases biphenyl-4-carboxylic acid sensitized to terbium luminescence in the presence of beta-lactamases, a paper-based photoluminescence test for terbium ions has been established to quickly and precisely identify drug-resistant bacteria [37]. Furthermore, mass spectrometry is quicker than conventional culture techniques and can rapidly detect the expression of beta-lactam enzymes [38]. Using computational biology techniques, 7 possible drug targets against multidrug resistant uropathogenic Escherichia coli strains that produce ESBL were found. In addition, the subcellular localization of 2 targets ECNA114_0085 and ECNA114_1060, cytoplasmic and periplasmic respectively, was predicted by computer simulation. These targets can be used to design drugs against ESBL-producing multidrug resistant uropathogenic E. coli [39]. A brief review of previous ESBL-positive urine culture results can help clinicians more precisely choose empirical antibiotic therapy and increase treatment success rates. These results are also very useful in clinical practice for predicting the pathogen identity of future UTIs [40].The CRISPR-Cas systems target and cleave DNA or RNA through base pairing guided by crRNA[41]. In the presence of the target, the bypass activity is activated to degrade the probe, thereby achieving high sensitivity and specificity in the detection of pathogenic bacteria [42]. Researchers made a detection system. It combines PCR and CRISPR-LbCas12a technology. This system can quickly and accurately detect Klebsiella and its ESBL-positive strains, making it appropriate for medical facilities without PCR equipment [43]. The efficacy of 2 novel culture systems—InTray COLOREX Screen/ESBL and Compact Dry—in identifying pathogens and ESBL-positive bacteria in urine samples was evaluated. These systems demonstrated not only exceptional sensitivity and specificity but were also cost-effective and user-friendly [44]. Using advanced detection technologies, we can better monitor antibiotic resistance. This helps clinicians choose the right antibiotics for treatment.
2. Gene Sequencing Technology for Beta-Lactamases
Gene sequencing technology can provide detailed gene sequence information and identify specific beta-lactamases genotypes. Gene sequencing technology plays an important role in understanding the diversity and resistance mechanism of beta-lactamases [45]. Gene sequencing can show the distribution of drug resistance genes in various geographical areas. For instance, community-acquired urinary tract pathogenic E. coli in Nouna primarily carry the blactX-M-1,3,15 genes, indicating that CefoTaXime-Munich ESBLs are relevant in local UTIs [46]. Research conducted in North India has revealed increased ESBL-positive rates for Klebsiella pneumoniae and E. coli, with the most prevalent ESBL types being CTX-M-1, CTX-M-15, TEM, and SHV [47]. A study in New Zealand found a genetic link between ESBL-producing E. coli from human clinical samples and those from the environment. It shows that rivers might be a source of ESBL-E. coli. The study especially noted the distribution of the ST131 sequence type and the bla(CTX-M-15) gene [48].
Furthermore, multidrug resistant bacteria and their resistance mechanisms were discovered using genome sequencing. For instance, a Provencencia strain that simultaneously produces bla(NDM-1), bla(VIEM-1), and bla(OXA-10) was first identified. Because this strain carries multiple beta-lactamases genes, it may be challenging to choose an antibiotic for clinical treatment [49]. Two strains of Providence were found to have several resistance genes, particularly the carbapenase gene and several beta-lactamases genes, according to whole genome sequencing [50].
Whole genome sequencing was employed to analyze ESBL-producing uropathogenic E. coli ST127 strain isolates obtained from patients across 5 hospitals in Armenia. Notably, these uropathogenic E. coli ST127 strain were found to produce ESBLs and carry genes encoding these enzymes, including bla(CTX-M-3), bla(CTX-M-236), and bla(TEM-1) [51]. Through a characteristic analysis of the plasmid carrying the blaNDM-1 gene extracted from Klebsiella pneumoniae, it was found that a metallic beta-lactamase known as NDM-1 can hydrolyze carbapenem antibiotics, rendering bacteria resistant to these potent drugs. In addition to the bla(NDM-1) gene, the IncX3 plasmid also harbors other resistance genes, including ble(MBL) and aph(3')-VI, indicating that the plasmid confers multidrug resistance [52]. Plasmid-mediated beta-lactamases genes, including blaCTX-M, blaSHV, and blaTEM, have been widely detected in multidrug resistant urinary tract pathogens [53]. E. coli strains that produce AmpC beta-lactamases are prevalent in UTIs and exhibit high levels of drug resistance. The common genes associated with this resistance include bla(CIT), bla(EBC), bla(FOX), and bla(DHA) [54].
The coexistence of NDM-1 and OXA-10 beta-lactamases genes in carbapenem-resistant Citrobacter DY2019 was also revealed for the first time by whole genome sequencing, suggesting that the strain spreads several resistance genes via various plasmids [55]. Multiple PCR identified the TEM, SHV, and CTX-M genes encoding broad-spectrum beta-lactamases in P. aeruginosa and K. pneumoniae isolated from individuals with UTIs, indicating the presence of multidrug resistance [56]. K. pneumoniae exhibits genotypic and phenotypic resistance incompatibility. Notably, some isolates harboring the blaKPC, blaIMP, blaVIM, or blaNDM-1 genes demonstrate phenotypic sensitivity to imipenem [57] (Fig. 1B).
THERAPEUTIC STRATEGIES OF BETA-LACTAMASE IN URINARY TRACT INFECTIONS
1. Beta-Lactam/Beta-Lactamases Inhibitor Combinations
Beta-lactamases inhibitors such as clavulanic acid, sulbactam, and tazobactam are widely used in clinical practice. These beta-lactam inhibitors significantly enhance the antibacterial activity of beta-lactam antibiotics by inhibiting most class A beta-lactam enzymes except carbapenase. However, they have limited inhibitory ability on class B, C, and D enzymes. In pediatric febrile UTIs, the study supported using methicillin and amoxicillin/clavulanate, or cefixime and amoxicillin/clavulanate together for treatment [58]. Cefixime and amoxicillin/clavulanic acid significantly increased cefixime's antibacterial activity against E. coli that produced ESBL, indicating that cefixime and amoxicillin/clavulanic acid could be a beneficial oral regimen for treating UTIs brought on by ESBL-positive E. coli [59]. By preventing the breakdown of beta-lactam antibiotics, piperacillin/tazobactam restored the antibacterial effect of ESBL, highlighting the significance of beta-lactam inhibitors in managing bacteria that produce ESBL [60]. In treating complex UTIs, ceftolozane/tazobactam has a highly effective antibacterial effect against multidrug resistant strains [61]. Novel beta-lactam/beta-lactamases inhibitor combinations, like ceftazidime-avibactam, ceftolozane-tazobactam, and imipenem-sulbactam, exhibited high activity and similar coverage against contemporary P. aeruginosa isolates. It is an important treatment option to combat the infection [62]. In addition, ETX1317 is a novel broad-spectrum beta-lactamases inhibitor. It binds to the beta-lactam active site and prevents it from hydrolyzing beta-lactam antibiotics. Compared with existing inhibitors like avibactam, it has stronger inhibition ability, a broader inhibition spectrum, and potential clinical application prospects [63]. In summary, beta-lactamases inhibitors are important in treating infections caused by multidrug resistant strains, especially against ESBL-producing bacteria and P. aeruginosa. These inhibitors restore and enhance the antimicrobial activity of beta-lactam antibiotics and reduce the development of resistance.
Avibactam and sulbactam are inhibitors of diazabicyclooctane enzymes. They do not have a beta-lactamases structure and are not easily hydrolyzed. They have a broader spectrum of beta-lactamases inhibition, and the inhibition effect is reversible. By creating a persistent covalent intermediate with class A beta-lactamases, particularly the CTX-M type, avibactam offers a novel solution to the issue of beta-lactamases-mediated resistance. This effectively inhibits enzyme activity by preventing the deacetylation route [64]. Avibactam is a novel non-beta-lactam beta-lactamase inhibitor that neutralizes ESBLs and AmpC beta-lactamases, restoring the efficacy of amicanam against drug-resistant pathogens. Azithromycin-avibactam is a new combination therapy against multidrug resistant Gram-negative bacteria, particularly strains that produce MBLs [65]. Phase III clinical trial results confirm that imipenem-cilastatin-sulbactam is effective and safe for treating complex UTIs and intraperitoneal infections. The drug's approval provides a new tool to combat multidrug resistant Gram-negative pathogens [66]. Avibactam and relebactam are non–beta-lactam beta-lactamases inhibitors. In treating multidrug resistant strains, they have significant antibacterial activity and exemplary safety. These inhibitors can restore the antibacterial effect of beta-lactam antibiotics and provide a new and effective method for clinical treatment.
Due to their simultaneous inhibition of serine beta-lactamases and MBLs, polycyclic borates, including bicyclic borates, exhibit promising therapeutic application prospects. The broad-spectrum inhibitory potential of developed bicyclic borates, like taniborbactam, to effectively inhibit class C beta-lactam enzymes, like AmpC, was discovered through enzyme kinetics and crystallographic studies. Nevertheless, the potent AmpC inhibition of these inhibitors is independent of their acyl-amino side chains [67]. Polycyclic borate beta-lactamases inhibitors have unique structures and inhibition mechanisms, which can provide a new therapy for overcoming antibiotic resistance. A new method for regaining the effectiveness of beta-lactamases antibiotics is provided by taniborbactam and QPX7728, which specifically bind to the active site of beta-lactamases through their bicyclic boric acid structure. This results in ultrabroad-spectrum inhibition of serine beta-lactamases and metallic beta-lactamases [68]. In complex UTIs, the combination of cefepime and taniborbactam has a remarkable antibacterial effect, which can overcome beta-lactamases-mediated resistance and reduce the bacterial load of the kidney [69]. When combined with cefepime or meropenem, taniborbactam can effectively target bacteria producing multiple beta-lactamases, providing new prospects for treating multidrug resistant Gram-negative infections [70]. Polycyclic borate beta-lactamases inhibitors can effectively restore the antibacterial effect of beta-lactam antibiotics and have significant clinical potential in treating complex infections. New beta-lactamases inhibitors (such as QPX7728, ETX0282, VNRX7145, etc.) combined with cephalosporins have excellent activity in vitro. These inhibitors contain boric acid groups and mainly inhibit ESBLs and AmpC enzymes. They bind to the beta-lactam active site, prevent the enzyme from hydrolyzing beta-lactam antibiotics, and enhance the antibacterial effect [71].
New beta-lactamases inhibitors have been designed and optimized to inhibit different types of beta-lactamases. The novel beta-lactamases inhibitors provide a variety of options and strategies for the treatment of antibiotic resistance. Fragment-based design techniques have been effectively used to create novel inhibitors that target Escherichia coli AmpC beta-lactamases, which show superior stability and binding ability in vitro compared to traditional antibiotics [72]. Furthermore, by blocking the activity of MBL, nitroxoline, and its derivatives restore the antimicrobial properties of beta-lactam antibiotics, particularly carbapenems, offering a novel approach to treating bacterial infections caused by MBL that are resistant to drugs [73]. Tebipenem is an oral carbapenem with antimicrobial activity and broad-spectrum activity comparable to intravenous carbapenems. It is stable in beta-lactamases and is a powerful tool for treating infection by multidrug resistant Gram-negative pathogens [74]. The researchers optimized the structure and found that 1,2, 4-triazole-3-thione derivatives could act as metal beta-lactamases inhibitors. It binds to metal beta-lactamases, reduces the activity of metal beta-lactamases, and restores the effectiveness of beta-lactamases antibiotics. These inhibitors considerably raised the sensitivity of resistant bacteria to meropenem when combined with it [75].
Furthermore, imipenem/sulbactam and meropenem/monobactam, 2 novel carbapenem/beta-lactamases inhibitor combos, may be appropriate choices for treating UTIs brought on by Enterobacteriaceae that are resistant to widely used antibiotics [76]. Levofloxacin and a carbon-dot coated CaCO₃ nanocore were used to create a novel antibiotic delivery system demonstrating outstanding antibacterial and antibiofilm action against multidrug resistant E. coli that produced broad-spectrum beta-lactamases. In addition to lowering the concentration of antibiotics needed, the nanocore technology also lowers the potential toxicity, offering new approaches and resources for treating bacterial illnesses resistant to several drugs [77]. The new beta-lactamases inhibitors have shown more significant inhibition against several beta-lactamases types through varied design and optimization (Table 1).
2. Optimization of Treatment Programs
In recent years, various antibiotics have demonstrated significant therapeutic potential against multidrug resistant bacterial infections. Optimizing treatment protocols can contribute to the reduction of antibiotic resistance development. The beta-lactam antibiotic cefmetazole works against bacteria by attaching itself to and preventing penicillin-binding proteins, which are necessary for the formation of bacterial cell walls. Research has demonstrated that cefmetazole is as effective as meropenem in treating invasive UTIs brought on by E. coli, both clinically and microbiologically [78]. Temoxicillin is a special beta-lactam antibiotic that is resistant to ESBL because it is not hydrolyzed by ESBL. Temoxicillin was proven to be as effective as carbapenem antibiotics in treating UTIs brought on by ESBL-E, making it a significant therapeutic option [79]. Amikacin is an aminoglycoside antibiotic that works against bacteria that manufacture beta-lactamases because it is unaffected by enzymes. Meropenem is a carbapenem antibiotic that has resistance issues even though it is stable against a lot of beta-lactamases. Amikacin is an alternative treatment option that is just as effective as meropenem, which is more commonly used, but has the benefit of being more affordable and convenient for outpatient treatment, particularly when treating UTIs brought on by beta-lactamases-producing E. coli [80]. Nitrofurantoin is the best choice for treating UTIs brought on by ESBL-producing bacteria, according to an analysis of the prevalence of ESBL-positive Enterobacteriaceae bacteria in Polonnaruwa District General Hospital and their susceptibility to widely used oral antibiotics [81]. In addition to identifying the most effective antibiotic options, it is crucial to optimize treatment strategies to minimize antibiotic exposure and mitigate the risk of resistance. Research indicates that shortening treatment cycles may serve as an effective approach to achieving this objective. In complex ESBL-EB UTIs, short-term (≤7 days) antimicrobial therapy was clinically as effective as long-term (>7 days) therapy. The 30-day all-cause mortality and reinfection combined measurements did not show any significant differences between the 2 regimens. To prevent needless long-term antibiotic use and the subsequent development of antibiotic resistance, short-term antimicrobial therapy may be a feasible and successful therapeutic option for complex ESBL-EB UTIs [82]. Step-down antibiotic treatment refers to a strategy in which patients are transitioned from intravenous antibiotics to oral antibiotics for continued treatment, contingent upon the stability of their condition and clinical response. The clinical outcomes of step-down treatment and primary oral antibiotic treatment were comparable in managing patients with ESBL-UTI. However, the duration of antibiotic use was significantly shorter in the group receiving oral therapy alone, thereby reinforcing the necessity to optimize antibiotic utilization [83]. Cefmezole, temoxicillin, amikacin, and nitrofurantoin have their own advantages in the treatment of complex infections. In-depth study of the mechanism of treatment and optimization of treatment programs can help solve the problem of antibiotic resistance.
3. Alternative Treatment Method
Vitamin C can down-regulate the expression of beta-lactamases coding genes and biofilm-related genes in uropathogenic E. coli, and reduce bacterial resistance to antibiotics. It can be used as an antibacterial and antibiological film agent, alone or in combination with antibiotics, can significantly improve UTIs in experimental rats [84]. 1, 8-cineole has antibacterial and antibiofilm activities on the biofilm of ESBL-producing strains, which can provide a new therapeutic strategy for the treatment of complex UTIs caused by uropathogenic E. coli produced by ESBL [85]. Recurrent UTIs brought on by K. pneumoniae that produce ESBL have also been successfully treated using oral freeze-dried fecal bacteria transplantation. For the treatment of recurring infections that are resistant to many drugs, the introduction of fecal bacteria transplantation may be a potential therapeutic strategy [86]. In conclusion, methods like vitamin C, 1, 8-cineole, and fecal bacterial transplantation have a lot of promise for treating UTIs and the medication resistance that goes along with them. These techniques offer a range of therapeutic alternatives in addition to improving the suppression of harmful microorganisms via several mechanisms (Fig. 1C).
PERSPECTIVE
The three-dimensional structure of beta-lactamases has been elucidated using high-resolution structural biology techniques, such as cryoelectron microscopy. These techniques reveal its catalytic mechanism and substrate specificity, thereby providing a theoretical foundation for the development of novel beta-lactamases inhibitors [87]. Furthermore, by analyzing the sequence and distribution of beta-lactamases genotypes, gene sequencing technology offers precise information for clinical treatment. This aids physicians in selecting more effective antibiotics, improving treatment success rates, and reducing antibiotic abuse [88,89].
The development of novel nanocarrier delivery systems is significant for beta-lactam antibiotics [90]. This system not only bypasses the degradation caused by beta-lactamases but also improves the targeted delivery and bioavailability of drugs. Additionally, it enhances the antibacterial effect through multiple mechanisms, reduces the development of drug resistance, improves treatment efficiency, and ensures human safety [91-93]. These advantages offer new strategies and tools for addressing multidrug resistant bacterial infections. For example, a new antibiotic delivery system combines a carbon-dot coated CaCO₃ nanocore with levofloxacin. This has shown excellent antibacterial and anti-biofilm activity against multidrug resistant and ESBLs producing E. coli [77].
CONCLUSIONS
Beta-lactamases play a central role in the antibiotic resistance associated with UTIs. They contribute to this resistance through mechanisms such as gene mutation, plasmid-mediated horizontal gene transfer, and the involvement of integrons. Specifically, a missense mutation in the beta-lactamases gene in patients with ESBL-positive UTIs enhances the resistance of E. coli to nitrofurantoin. Integrons facilitate the horizontal gene transfer of ESBL-encoding genes, thereby increasing bacterial resistance; the blaCTX-M-15 gene, carried by plasmids, further elevates the resistance of E. coli to beta-lactam antibiotics.
Strengthening multidisciplinary cooperation and formulating more scientific and rational guidelines for antibiotic use, in conjunction with public health policies and clinical practice, is essential to mitigate the further development of drug resistance. This approach will lead to safer and more effective treatment options for patients with UTIs. Furthermore, the application of machine learning and artificial intelligence technologies to predict and monitor trends in drug resistance will facilitate timely adjustments to treatment strategies and optimize antibiotic use. In addition to traditional antibiotic therapy, alternative treatments such as vitamin C and fecal microbiota transplantation should be actively explored. These methods not only enhance the inhibition of pathogenic bacteria through diverse mechanisms but also provide a wider array of treatment options.
Notes
Funding/Support
This study was supported by the regional innovation cooperation project of Sichuan Province (Grant No. 23QYCX0136).
Conflict of Interest
DF, a member of the Editorial Board of Urogenital Tract Infection, is a co-corresponding author of this article. However, he played no role whatsoever in the editorial evaluation of this article or the decision to publish it. The other authors have nothing to disclose.
Acknowledgments
We appreciated the Figdraw (www.figdraw.com) and Chengdu Basebiotech Co.,Ltd for their assistance in drawing and data process.
Author Contribution
Conceptualization: FS, DL, RW, DF. Data curation: FS, DL, JW, ZT, ZW, WW. Formal analysis: FS, DL, JW, ZT, ZW, WW. Methodology: FS, DL, JW, ZT, ZW, WW. Project administration: RW, DF. Visualization: FS, DL, JW, ZT, ZW, WW. Writing - original draft: FS, DL. Writing - review & editing: FS, DL, RW, DF.

