Mortality and Risk Factors for Emphysematous Pyelonephritis in Korea: A Multicenter Retrospective Cohort Study
Article information
Abstract
Purpose
Emphysematous pyelonephritis (EPN) is a life-threatening disease requiring immediate treatment. This multicenter retrospective cohort study aimed to analyze the mortality rate and risk factors associated with EPN.
Materials and Methods
Between January 2011 and February 2021, 217 patients diagnosed with EPN via computed tomography who visited 14 teaching hospitals were retrospectively analyzed. Clinical data, including age, sex, comorbidities, Huang and Tseng classification, hydronephrosis, acute kidney injury, blood and urine tests, surgical interventions, percutaneous drainage, and conservative treatments, were compared between the survival and death groups. Risk factors for mortality due to EPN were analyzed using univariate and multivariate methods.
Results
The mean age of survivors and deceased patients was 67.8 and 69.0 years, respectively (p=0.136). The sex distribution (male/female) was 48/146 and 8/15, respectively (p=0.298). Of the 217 patients, 23 died, resulting in a mortality rate of 10.6%. In univariate analysis, the Huang and Tseng classification (p=0.004), platelet count (p=0.005), and acute kidney injury (p=0.007) were significantly associated with mortality from EPN. In multivariate analysis, only the Huang and Tseng classification (p=0.029) was identified as a risk factor. Mortality rates according to the Huang and Tseng classification were as follows: class I (5.88%), class II (7.50%), class IIIa (14.28%), class IIIb (25.00%), and class IV (23.07%).
Conclusions
EPN is associated with a high mortality rate. Among various clinical factors, the Huang and Tseng classification was the most significant indicator for predicting mortality.
HIGHLIGHTS
This multicenter study analyzed 217 patients with emphysematous pyelonephritis to identify mortality-related risk factors. Huang and Tseng classification was significantly associated with mortality, highlighting the need for early risk stratification.
INTRODUCTION
Emphysematous pyelonephritis (EPN) is a severe and life-threatening kidney infection caused by gas-forming bacteria, leading to gas accumulation in the renal parenchyma and surrounding tissues [1]. This condition primarily occurs in patients with specific risk factors and may lead to serious complications. Due to its rapid progression and high mortality rate, early diagnosis and appropriate management are crucial [2,3].
Treatment approaches for EPN can vary significantly across countries due to differences in healthcare systems, resources, policies, and cultural backgrounds [4,5]. Various factors, including healthcare accessibility, antibiotic usage and resistance, prescription practices, preventive measures such as vaccination, and investment in research and development, play a crucial role in shaping the management and outcomes of infectious diseases worldwide [6]. Understanding these variations is crucial for developing effective infection control strategies tailored to the unique characteristics of each healthcare setting.
Among various classification systems for EPN, the Huang and Tseng classification system is one of the most widely used and clinically significant methods for assessing disease severity. This system categorizes EPN into four classes based on the extent of gas formation and tissue involvement, providing critical prognostic information and guiding treatment decisions. Higher classification levels are associated with increased mortality, often necessitating more aggressive interventions, including nephrectomy [1].
The prognosis of EPN varies widely. In the 1980s, EPN was associated with excessively high mortality rates of approximately 40%. Although advancements in diagnostic imaging, antibiotic therapy, and management strategies have been made, reported mortality rates remain significant, ranging from 7% to 25% [7,8]. Early diagnosis and prompt, aggressive treatment are essential to improving patient outcomes [9]. Given the life-threatening nature of EPN, immediate intervention is crucial [10]. Although several studies have investigated patients with EPN, most had a relatively small sample size due to the rarity of the disease, and large-scaled or multicenter studies are still lacking. This multicenter retrospective cohort study aimed to analyze the mortality rate and identify risk factors for EPN, with a particular focus on the prognostic significance of the Huang and Tseng classification system.
MATERIALS AND METHODS
Patients diagnosed with EPN by using abdominal computed tomography (CT) between January 2011 and February 2021 at 14 Korean institutes were reviewed.
Patients who could influence the results, such as those with recent abdominal trauma, a history of fistula between the digestive and urinary tracts, or those who had undergone kidney-related surgery or procedures, were excluded.
Patient characteristics, including age, sex, comorbidities (diabetes mellitus, hypertension, stroke, and chronic kidney disease), acute kidney injury, blood and urine tests (performed using samples collected at the initial visit), Huang and Tseng classification, treatment modalities (medical, minimally invasive, and surgical therapies), and treatment outcomes (sepsis, septic shock, or mortality), were analyzed. Causative organisms and antibiotic resistance profiles were assessed through urine cultures, including the presence of extended-spectrum β-lactamase-producing bacteria. Some hospitals did not perform blood cultures at the initial visit. Additionally, among those that did, there were inconsistencies in the timing of testing and variations in the results. Consequently, blood culture data were excluded from the analysis.
EPN was defined as gas accumulation in the collecting system, renal parenchyma, or perinephric or pararenal spaces as detected on CT. The Huang and Tseng classification system was utilized as follows: class I, gas confined to the collecting system; class II, gas confined to the renal parenchyma alone; class IIIa, perinephric extension of gas or abscess; class IIIb, extension of gas beyond Gerota fascia; and class IV, bilateral EPN or EPN in a solitary kidney (Fig. 1). Medical management included supportive therapies such as hemodynamic stabilization, metabolic control, and antibiotic therapy. Minimally invasive management included percutaneous catheter drainage or placement of an indwelling ureteral stent. Surgical management involved open drainage or nephrectomy. Sepsis and septic shock were defined according to the third international consensus definition of sepsis and septic shock (Sepsis-3) [11-13].
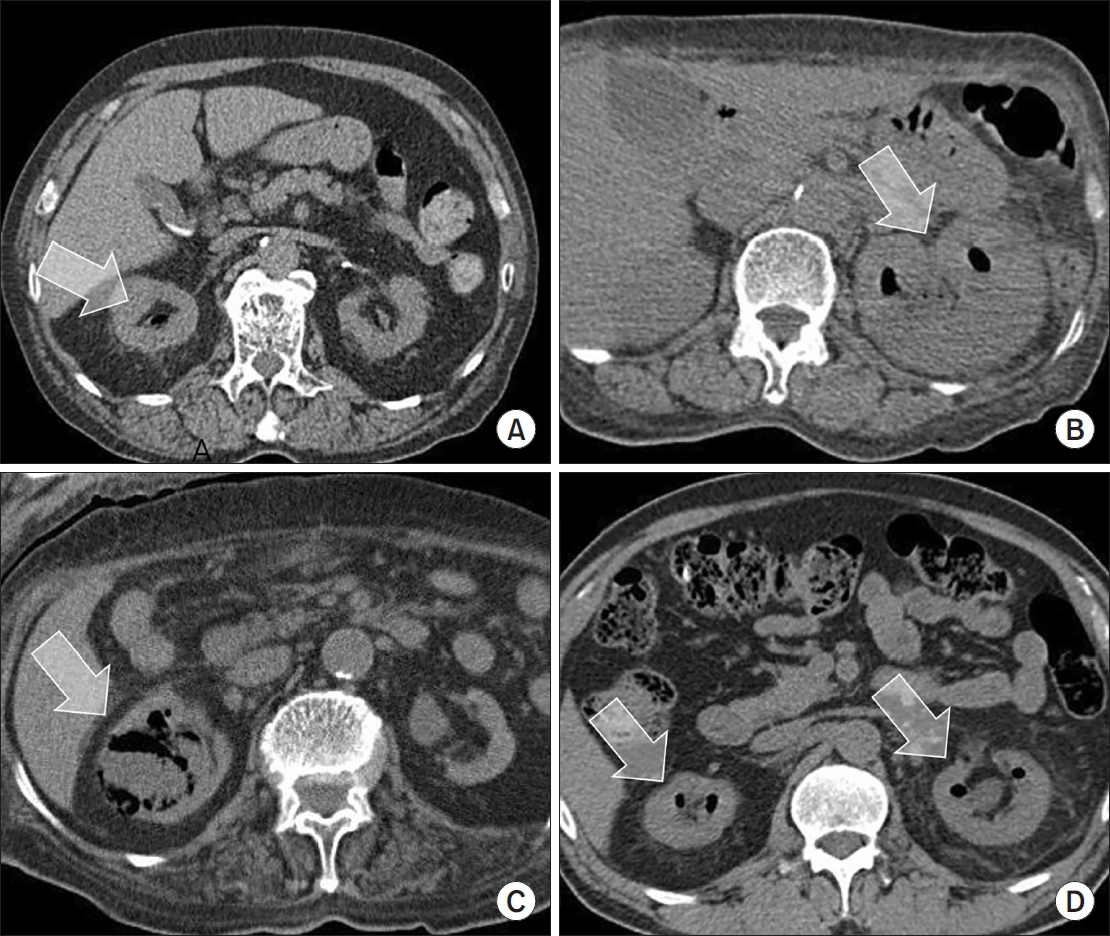
Huang and Tseng classification system. (A) Class I, gas confined to the collecting system. (B) Class II, gas confined to the renal parenchyma alone. (C) Class III, perinephric extension of gas or abscess, extension of gas beyond Gerota fascia. (D) Class IV, bilateral emphysematous pyelonephritis or emphysematous pyelonephritis in a solitary kidney. Arrows indicate areas of gas formation within the renal parenchyma or collecting system, which is a characteristic finding of emphysematous pyelonephritis.
Descriptive analyses were conducted for all categorical and continuous variables. Treatment modalities, outcomes, and patient groups were compared using Pearson chi-square test. All statistical analyses were performed using IBM SPSS ver. 18.0 (IBM Co., Armonk, NY, USA).
RESULTS
Of the 217 patients included in the study, 23 died, resulting in a mortality rate of 10.6%. The age (mean± standard deviation) of survivors and deceased was 67.8± 12.9 years and 69.0±11.6 years, respectively (p=0.136). Among survivors, the male-to-female ratio was 48:146, while among deceased patients, it was 8:15 (p=0.298). In the group of patients who died, there were more patients with a high Huang and Tseng classification than those of patients who survived (p=0.004). Platelet count was significantly lower in the deceased patient group than in the survivor group (p=0.005). The proportion of patients with acute renal failure during treatment of EPN was higher in the deceased patient group than in the survivor group (p=0.007). There were no significant differences between the deceased patients and survivors with respect to age, comorbidities, causative organisms, leukocyte count, serum creatinine level, urinary tract obstruction, and treatment modalities (p>0.05) (Table 1).
Urine culture analysis revealed positive growth in 143 patients. Escherichia coli was the predominant pathogen, identified in 113 patients (79.0%), followed by Klebsiella pneumoniae in 19 patients (13.3%). The remaining 11 patients (7.7%) had infections caused by various other microorganisms, including Enterococci, Pseudomonas, Candida, Proteus, Morganella, Staphylococcus, and polymicrobial infections.
The incidence of sepsis and septic shock was higher in the deceased patient group than in the survival group. Sepsis was observed in 95 patients (49%) in the survival group and 21 patients (91%) in the deceased group. Similarly, septic shock occurred in 42 patients (22%) in the survival group and 15 patients (65%) in the deceased group.
Univariate analysis revealed that the Huang and Tseng classification (p=0.004), platelet count from routine blood tests (p=0.005), and acute kidney injury (p=0.007) were significantly correlated with mortality from EPN. In the multivariate analysis, only the Huang and Tseng classification (p=0.029) was a risk factor for death from EPN (Table 2).
Mortality rates according to the Huang and Tseng classification were class I (5.9%), class II (7.5%), class IIIa (14.3%), class IIIb (25.0%), and class IV (23.1%) (Table 3).
DISCUSSION
EPN is a rare and life-threatening condition due to septic complications, with mortality rates reaching up to 80% if not promptly diagnosed and treated [7,8,14,15]. The demographic characteristics of patients with EPN in our study aligned with previous research findings. Most patients were women (74.2%), and E. coli was the predominant causative pathogen, followed by other gram-negative bacteria. Consistent with earlier studies, diabetes mellitus was the most common risk factor for EPN and the predominant underlying disease in the present study cohort (82.0%) [7,16,17]. Obstructive uropathy (30.4%) was also common among the patients, which is consistent with the findings of other studies.
The mortality rate of EPN in Korea is reported to be approximately 10%, while globally, it varies ranging from 10% to 40% [7,8]. Several factors contribute to these differences in mortality rates between Korea and other countries [4]. The first factor is ‘Healthcare System Accessibility.’ South Korea has a unique National Health Insurance (NHI) system, with a single insurer, the National Health Insurance Corporation, covering almost all citizens. This NHI system allows patients considerable freedom in choosing their service providers [18,19]. The treatment outcomes are optimal because prompt antibiotic treatment and surgical intervention are performed when necessary. However, antibiotic resistance and inadequate treatment approaches may be challenging in other parts of the world. The second factor is ‘Chronic Disease Management.’ In Korea, under the NHI system, health outcomes are relatively successful along with preventive healthcare for everyone in the country [18]. Most patients with chronic diseases are relatively well managed, which may lead to a low incidence of EPN in Korea. However, the mortality rate may be higher in some countries due to a high prevalence of patients with underlying conditions such as diabetes. The third factor is ‘Infrastructure and Equipment.’ Korea has a relatively well-developed medical system that enables early diagnosis and treatment [20]. This robust healthcare infrastructure facilitates precise diagnosis and effective management of patients with EPN. However, in some countries, such infrastructure may be lacking, potentially leading to higher mortality rates. In addition, other factors, including ethnicity, and regional characteristics might have had impacts.
Severe inflammatory responses caused by EPN can impact both platelet production and renal function. In sepsis, excessive platelet consumption may lead to thrombocytopenia, increasing the risk of bleeding and related complications. Simultaneously, EPN-induced renal tissue necrosis and inflammation can cause acute kidney injury, leading to electrolyte imbalances, toxin accumulation, and systemic complications [1]. Given the interrelation between thrombocytopenia and renal failure, continuous monitoring and appropriate management of platelet count and renal function are crucial for improving patient outcomes.
The Huang and Tseng classification is a tool for systematically assessing the severity of EPN, and has a significant impact on mortality [1]. This classification divides EPN into four grades, each graded according to the clinical prognosis and treatment approach. Class I is characterized by emphysematous changes confined to the kidneys. Mortality is low (10% or less), and recovery is usually possible with antibiotic treatment. At this stage, prompt diagnosis and treatment significantly improve patient prognosis. Class II is characterized by emphysema spreading to tissues surrounding the kidney, and mortality increases to an intermediate level (10%– 30%). Surgical intervention along with antibiotics may be required at this stage, and the risk of complications may increase depending on the patient's condition. Class III is characterized by emphysema spreading outside the kidney, and the mortality rate increases to more than 30%. Urgent surgical intervention is often required and a delay in treatment may further reduce the possibility of survival. Class IV is characterized by multiple organ failure or severe sepsis and has a high mortality rate. Owing to the complexity of treatment and the significant impact of a patient's overall condition on prognosis, prompt diagnosis and comprehensive management are essential. Similarly, our study demonstrated a significant association between the Huang & Tseng classification system and mortality, with higher mortality rates observed in class IIIB and class IV patients. These findings confirm the usefulness of the Huang & Tseng classification system consistent with previous research. The Huang and Tseng classification system is crucial for predicting the mortality rate of patients with EPN and aids in establishing management strategies and treatment priorities for each grade. Accurate assessment of a patient's condition and appropriate treatment based of this classification significantly enhance survival outcomes.
This study has limitations inherent to retrospective studies. The treatment protocols may vary across institutions, which may result in inconsistent data. The Huang and Tseng classification was initially determined by radiologists at the time of the patient's visit, followed by a secondary assessment by urologists. However, in cases where radiologists did not apply the Huang and Tseng classification during their evaluation, the classification was performed solely by urologists. This limitation may have led to classification discrepancies, introducing a potential source of bias. Our results cannot represent the EPN mortality rate in Korea. However, this study was conducted in 14 institutions nationwide, which enhances its representativeness. Additionally, this study was conducted with a larger number of patients compared to other studies.
CONCLUSIONS
EPN is associated with a high mortality rate. The mortality rate from EPN was 10.6% in Korea. Among various clinical factors, the Huang and Tseng classification emerged as the most significant predictor of mortality. Patients with high Huang and Tseng classification had the high risk of mortality, and required immediate and aggressive treatment. A larger prospective cohort study is required to support our findings.
Notes
Funding/Support
This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Research Ethics
This retrospective cohort study was reviewed and approved by the Institutional Review Board of Seoul Medical Center (SEOUL 2021-05-006-002).
Conflict of Interest
SKC, a member of the Editorial Board of Urogenital Tract Infection, is the corresponding author of this article. However, he played no role whatsoever in the editorial evaluation of this article or the decision to publish it. The other authors have nothing to disclose.
Author Contribution
Conceptualization: SKC; Data curation: SB, JBC, YK, JMC, JHS, JHJ, HC, THK; Formal analysis: ECH, SKC; Methodology: SKC; Project administration: SKC; Visualization: SKC; Writing - original draft: SKC; Writing - review & editing: JWL, SIJ, ECH, JC, WBK, JSH.



