Case of Life-Threatening Pneumonia during the Treatment of a Patient with Acute Bacterial Prostatitis
Article information
Abstract
Acute bacterial prostatitis is an acute urinary tract infection associated with a bladder outlet obstruction or an immunosuppressed state. A 51-year-old man patient visited the hospital with fever, chills, and acute urinary retention that started the day before his visit after consuming a significant amount of alcohol. Conservative treatments, including catheterization for urinary drainage and antibiotics, were performed. On the third day of treatment for acute prostatitis, he complained of dyspnea. The level of oxygen differentiation was reduced significantly, and the tracheal insertion and ventilator were maintained after the radiological examination. The ventilator was discontinued, and the prostate abscess was operated on the eighth day of hospitalization. He was discharged without complications. This paper reports a case of life-threatening pneumonia and a prostate abscess during the treatment of a patient with acute bacterial prostatitis with a review of the relevant literature.
Acute bacterial prostatitis (ABP) is an acute urinary tract infection associated with a bladder outlet obstruction or an immunosuppressed state that can occur in males of any age. The progression of ABP can occur in a variety of ways depending on the patient’s chronic disease and treatment time [1]. A prostate abscess is a rare complication of the treatment for acute prostatitis [2]. The most common strains of this disease are Escherichia coli (E. coli). Klebsiella pneumoniae (K. pneumoniae), and Staphylococcus [3-5]. K. pneumoniae is a member of the Enterobacteriaceae family and is an opportunistic pathogen that causes pneumonia, bacteremia, and urinary tract infections depending on the patient’s health condition [6].
This paper reports a case of life-threatening pneumonia and a prostatic abscess that occurred during the treatment of a patient with ABP caused by K. pneumoniae with a review of the relevant literature.
CASE REPORT
A 51-year-old man had a prior medical history of cholecystectomy caused by a gallbladder stone without chronic disease, such as diabetes mellitus and hypertension. He presented at the emergency department with fever, dysuria, hesitancy, and acute urinary retention one day ago after consuming a considerable amount of alcohol.
The laboratory data revealed the following serum levels: white blood cells (WBCs) of 13.36×103/μl, 1.40 mg/dl creatinine, 127 mmol/L sodium, C-reactive protein of 30.19 mg/dl, erythrocyte sedimentation rate of 120 mm/hr, and WBC and red blood cells of many/high-pass filter in the urine. The initial chest posteroanterior (PA) revealed no active lung lesion (Fig. 1A). Computed tomography (CT) of the abdomen and pelvis showed no gall bladder and an enlarged prostate (Fig. 2A). He underwent a 16 Fr foley catheter and received sodium replacement and Pipe-racillin/Tazobactam 4.5 g IV tid for three days. On the second day of hospitalization, transrectal ultrasound showed no evidence of a prostate abscess except for an enlarged prostate (Fig. 3). On the third day of hospitalization, the patient complained of dyspnea. His general condition gradually deteriorated, and his blood pressure, heart rate, respiratory rate, and body temperature were 96/47 mmHg, 142/min, 35/min, and 38.6°C, respectively. The chest X-ray (Fig. 1B) showed newly appeared diffuse patchy and nodular opacities throughout both lungs. The common blood counts and arterial blood analysis revealed a platelet count of 16×103 counts/mm3, partial pressure of oxygen level of 59.6 mmHg, partial pressure of carbon dioxide of 30.2 mmHg, and potential hydrogen of 7.43. A CT scan of the chest showed dense consolidation in both lobes (Fig. 4). He was under severe respiratory distress, and his blood pressure decreased. Subsequently, a vasopressor was used to increase his blood pressure. The antibiotic was changed (meropenem 2 g for three days), and a ventilator was inserted in the intensive care unit. The culture of blood and urine yielded K. pneumoniae (Table 1). The patient’s conscious state changed, but there was no significant lesion on the magnetic resonance imaging scan of the brain.
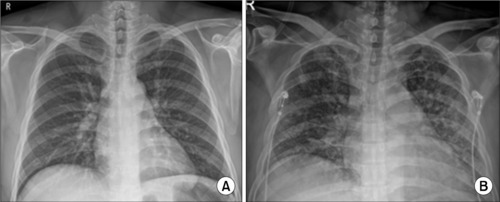
(A) On the first day. Chest posteroanterior (PA): no active lung lesion. (B) On the third day. Chest PA: newly appeared diffuse patchy and nodular opacities throughout both lungs.
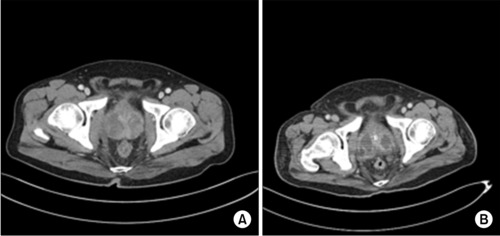
In the contrast-enhanced com-puted tomography scan. (A) The axial view showed prostatic enlargement with heterogeneous enhancement on the admission day. (B) The axial view demonstrated multiple loculated fluid collections and lobulated lesions in the prostate on the seventh day after hospitalization.

On the second day, transrectal ultrasound revealed a prostate volume of 152 gm with central gland enlargement and calcifications, and heterogeneous parenchymal echoes were observed in both peripheral zones.
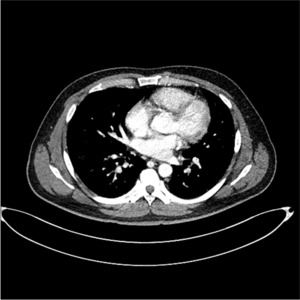
Computed tomography scan of the chest showed dense consolidation in both lobes (pneumonia with acute respiratory distress syndrome).
The patient’s blood pressure was maintained, and the vasopressor was gradually decreased. On the sixth day of admission to the intensive care unit, the vasopressor was discontinued. A CT scan of the abdomen and pelvis demonstrated multiple loculated fluid collections and a lobulated lesion in the prostate (Fig. 2B). The prostate abscess was drained by transurethral unroofing of the prostate on the eighth day of admission to the intensive care unit (Fig. 5). The patient’s spontaneous respiration was maintained, and the ventilator was discontinued on the 10th day. The patient was then transferred to the general ward on the 13th day. The pathology result of the prostate was suppurative inflammation. He was discharged with full recovery after 19 days of admission. Four weeks after discharge, transrectal ultrasound revealed a reduced prostate volume (Fig. 6). He did not complain of voiding symptoms, and no complications had occurred nine months after discharge. Authors received informed consent for the use of the patients’ photographs or other information through the publication.
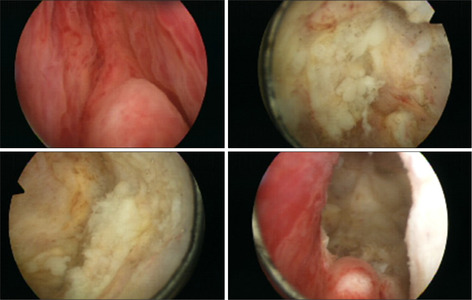
On the eighth day of admission to the intensive care unit, transurethral unroofing of the prostate abscess.
DISCUSSION
ABP is an acute urinary tract infection that can happen at any age but occurs frequently among patients with immunocompromised status and becomes increasingly common with age [1,7]. ABP has a variety of outcomes depending on the patient’s condition or chronic disease, and a prostate abscess may occur [2,5]. Among various chronic diseases, diabetes mellitus is one of the risk factors for a prostate abscess. Delaying the diagnosis and treatment of prostate abscess increases the mortality rate [2,7].
Symptoms of prostate abscess are voiding symptoms, including dysuria, tenesmus, and urinary retention accompanied by fever or perineal discomfort [8]. In this case, the patient visited the emergency room with fever, dysuria, and acute urinary retention.
The strains causing prostatic abscesses include E. coli, K. pneumoniae, and Staphylococcus [3-5]. A previous study reported E. coli in four cases, K. pneumoniae in three cases, and Staphylococcus aureus in one case [5]. In the present case, K. pneumoniae was identified in the urine and blood culture.
K. pneumoniae is an opportunistic pathogen that causes pneumonia (rare primary pneumonia but nosocomial pneumonia) and hospital-acquired urinary tract infections [7,9]. The occurrence of bacteremia and urinary tract infections depends on the patient’s health condition [6].
One study reported a 60-year-old man with diabetes, previously treated for ABP, who had developed a prostate abscess caused by K. pneumoniae, complicating septic pulmonary emboli and meningitis [7]. Acute respiratory distress syndrome (ARDS) is a life-threatening condition caused by bacteremia or viruses and can result from infectious diseases that have worsened [10].
In the present case, a patient with no diabetes mellitus used the appropriate antibiotics (Piperacillin/Tazobactam) according to the results of a previous study [5]. He also had no respiratory symptoms at the time of hospitalization and no abnormality in the chest PA examination. He was treated immediately on the day the symptoms appeared. On the third day of hospitalization, he deteriorated rapidly and had ARDS. He recovered completely through conservative treatment with vasopressors, ventilators, transfusions, and antibiotic changes.
In conclusion, physicians should consider the treatment of patients with ABP who do not have chronic diseases, such as diabetes mellitus. Despite using the appropriate antibiotics when the symptoms were recognized, K. pneumoniae from acute prostatitis spread to multi-organs, including the lungs and brain. ARDS could not be suppressed, and the symptoms intensified [7]. Therefore, if a patient is experiencing difficulty breathing or other symptoms, physicians should check the lesions rapidly and treat them through chest and abdominal computed tomography, even before the bacteria can be identified.
Notes
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.
FUNDING
No funding to declare.
AUTHOR CONTRIBUTIONS
K.K.P. participated in data collection and wrote the manuscript. S.D.K., Y.J.K. participated in the study design. J.S.H. participated in the study design and coordination and helped to draft the manuscript. All authors read and approved the final manuscript.


